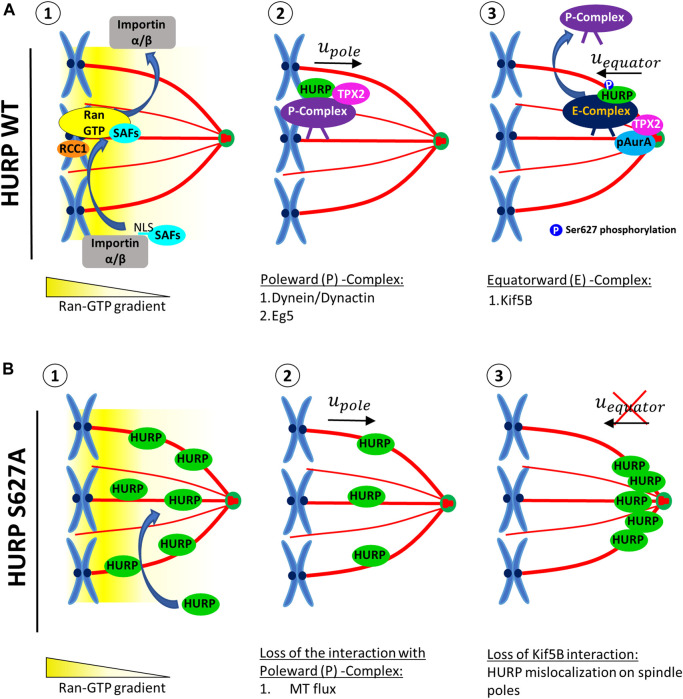
FIGURE 7.
A proposed model for the localization of HURP in the vicinity of chromosomes on the metaphase spindle, involving a two-step process. (A) In the presence of high Ran-GTP concentration (1), SAFs including HURP are released from the inhibitory binding of Importins α/β near the chromosomes. HURP, along with other MAPs and motors, bind to MTs and forms the Poleward Complex (P-Complex). The P-Complex includes HURP, TPX2, Dynein/Dynactin, and Eg5. It is then transported towards the spindle pole in a Dynein/Dynactin-dependent manner (2). As the P-Complex reaches the intermediate zone, it interacts with the active Aurora A kinase (pAurA). TPX2 promotes hyperactivation of Aurora A, leading to the phosphorylation of HURP, at least at the Ser627 residue. This phosphorylation event disrupts the Poleward Complex and a new HURP complex is formed (Equatorward (E)-Complex), now containing at least a kinesin, such as Kif5B, which moves towards the equator (3). (B) The HURP S627A mutant molecules can bind to MTs independently of the Ran-GTP gradient due to the loss of inhibitory binding of Importin β to HURP (1). These mutant molecules bound to the spindle move towards the poles, possibly due to MT flux (2). However, since the mutant HURP molecules are unable to be phosphorylated at Ser627 by Aurora A and cannot interact with Kif5B, they cannot form an E-Complex and instead accumulate at the spindle poles (3).