Fig. 3 |. Platelet sGC influences the release of ANGPT1.
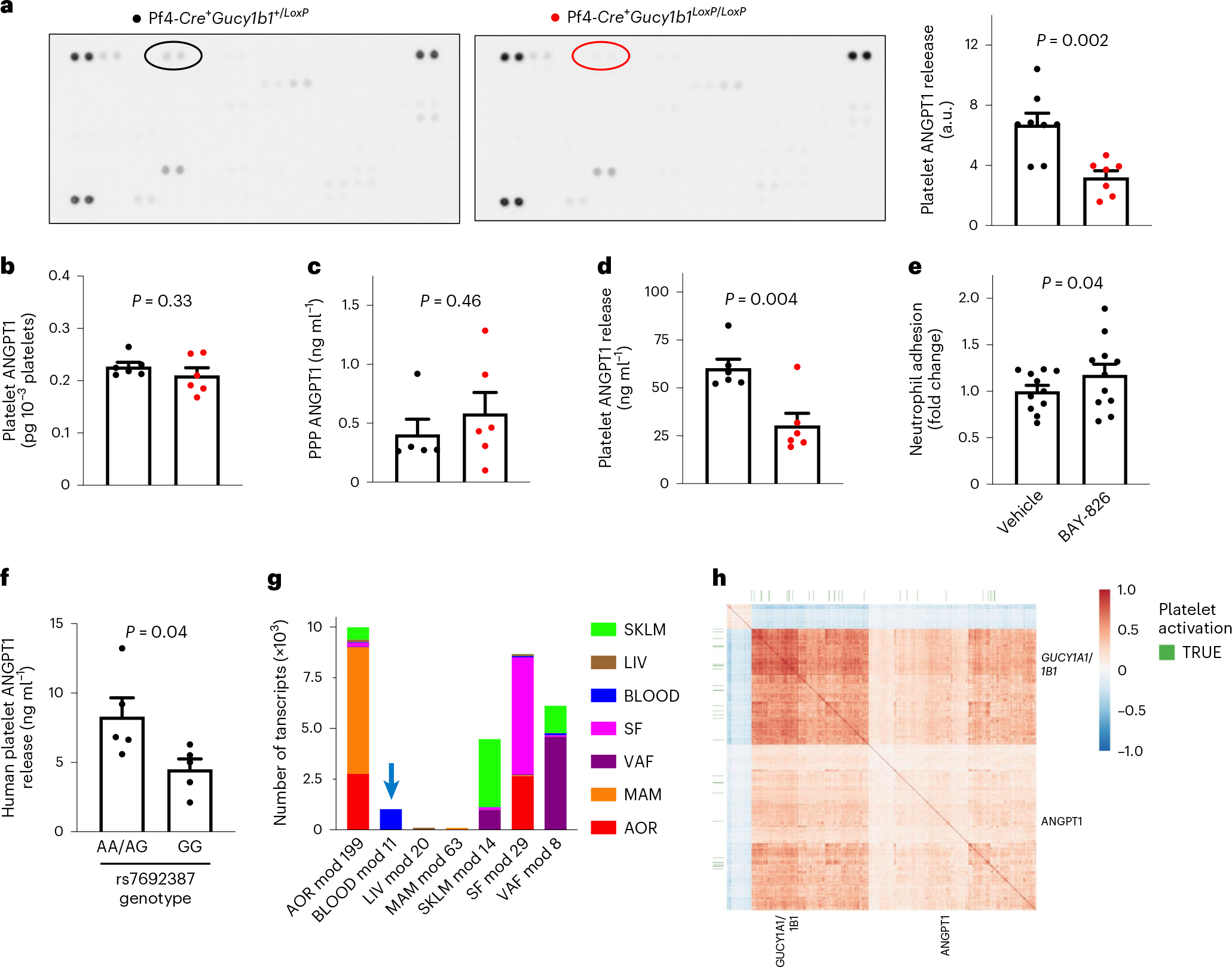
a, Left, identification of ANGPT1 (encircled) as differentially released protein from activated Pf4-Cre+Gucy1b1+/LoxP and Pf4-Cre+Gucy1b1LoxP/LoxP platelets. Right, quantification of ANGPT1 signal in 8 Pf4-Cre+Gucy1b1+/LoxP and 7 Pf4-Cre+Gucy1b1LoxP/LoxP mice. Two-sided unpaired t-test. b–d, Quantification of platelet ANGPT1 content (n = 6 independent animals) (b), PPP ANGPT1 (n = 5 and 6 independent animals, respectively) (c) and released ANGPT1 as determined in 6 independent animals per group by ELISA (d). Two-sided unpaired t-test. e, WT neutrophil adhesion to WT ECs after incubation with supernatant of activated WT platelets in the absence and presence of the Tie2 inhibitor BAY-826 (0.5 μM). Two-sided paired t-test on n = 11 sample pairs derived from independent animals. f, Platelet ANGPT1 release in five humans carrying the GUCY1A1 (rs7692387) non-risk (AA, AG genotype) allele and five homozygous carriers of the risk allele (GG genotype). Each symbol represents one individual. Two-sided unpaired t-test. Data are the mean ± s.e.m. g, STARNET coexpression modules containing ANGPT1 from multitissue RNA-seq sampling of approximately 600 patients with CAD. The arrow denotes coexpression module 11 from whole-blood samples (BLOOD). h, Heatmap of Pearson’s correlation coefficients of genes in coexpression module 11, showing positive correlation of ANGPT1, GUCY1A1 and GUCY1B1 along with enrichment for platelet activation genes (false discovery rate = 6.863 × 10−13, Enrichr, KEGG pathway). AOR, aorta; LIV, liver; MAM, mammary artery; SF, subcutaneous fat; SKLM, skeletal muscle; VAF, visceral fat.
