Figure 5:
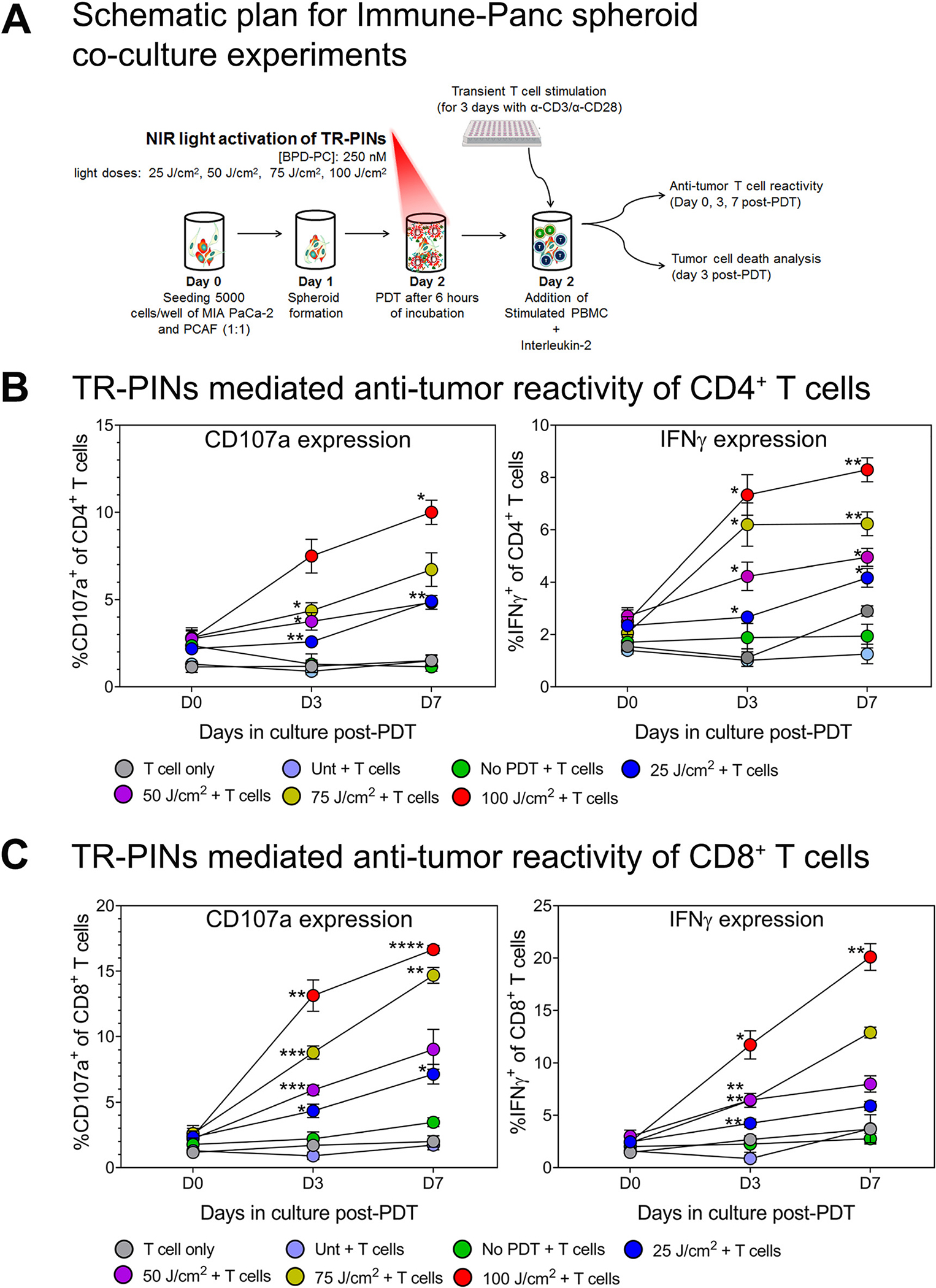
TR-PINs mediated priming of antitumor T cell reactivity in Immune-Panc spheroid co-cultures.
(A) MIA PaCa-2 and PACFs were cultured and allowed to grow for 48 h before co-culture with peripheral blood mononuclear cells (PBMC).PBMC were seeded in 6-well plates with plate-bound anti-CD3 (overnight), anti-CD28, and IL-2, and T cells were allowed to proliferate for three days before addition to 3D spheroid cultures. This was done using a previous protocol with slight modifications [57, 58]. Once the spheroids were exposed to varying light doses, PBMC consisting mainly of mildly stimulated T cells were added to the cultures and allowed to remain for seven days. T cell priming was evaluated at day 3 and 7 post-PDT by analyzing the surface expression of degranulation marker CD107a and intracellular expression of INFγ. Also, in the same cultures, spheroid cell death was evaluated by flow cytometry analysis on day 3. The expression of CD107a and INFγ from day 0 in culture to day 7 was evaluated in (B) CD4+ T cells and (C) CD8+ T cells using multi-color flow cytometry. Data are means ± SEM from three to four independent experiments done in duplicate. Statistical significance was determined by a one-way ANOVA and Tukey’s posthoc test. Asterisks denote statistical significance (*P < 0.05, **P < 0.005, ***P < 0.0005). The NIR photodynamic activation regimen consisted of 690 nm light irradiation with 25 or 50 or 75 or 100 J/cm2 at 150 mW/cm2. TR-PINs (BPD-PC equivalent) were used at a concentration of 250 nM. All experimental conditions shown involved co-cultures Immune-Panc spheroids along with the addition of the BPD-containing TR-PINs, except for one condition with PBMC only (T cell only), and another with untreated Immune-Panc spheroids with added immune cells but no photosensitizer (Unt + T cells).
