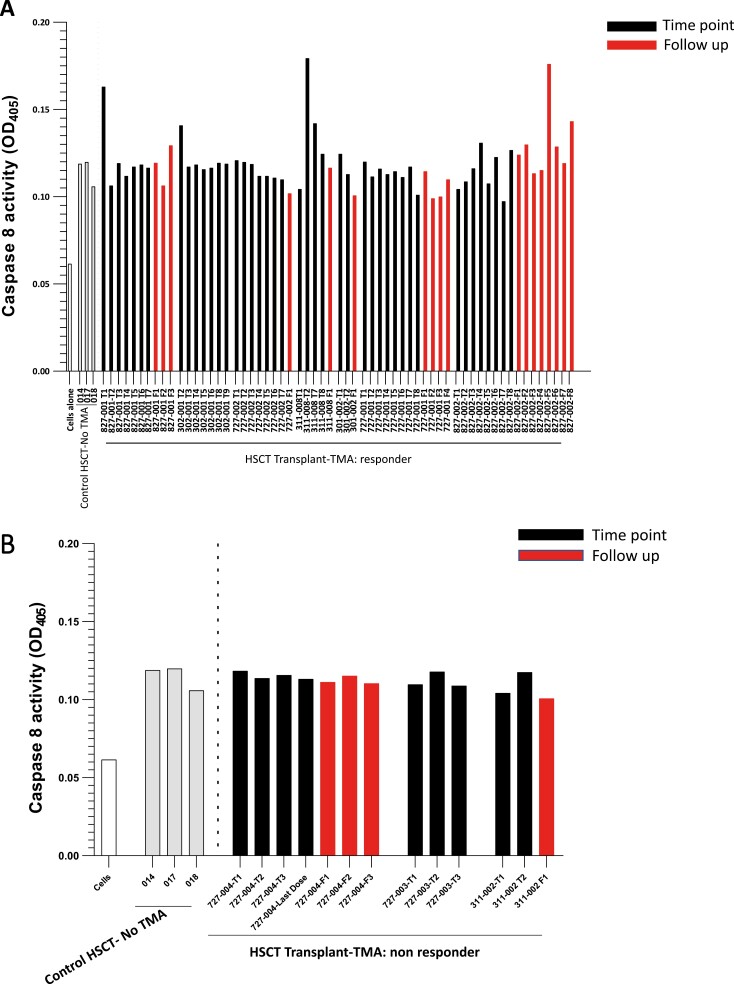
Figure 1.
Caspase 8 activation I MVEC by plasmas from acute TA-TMA patients pre-, during, and post-narsoplimab administration. Cultures of primary human neonatal dermal microvascular endothelial cells (MVEC) were exposed to plasma (2% v/v) from patients with acute TA-TMA collected prior to (black bars, T1), during (subsequent black bars), and following (red bars) cessation of narsoplimab administration. These subjects were part of clinical trial NCT02222545. Clinical response was defined as described in the text. The grey bars represent plasmas from three patients who underwent an allogeneic stem cell transplant but did not develop a TMA. They were collected as part of an observational cohort (NCT02604420) at about day 120 post-transplant, the median time for TA-TMA development. A. Serial samples from 7 of the 9 clinical responders to narsoplimab are illustrated. B. Serial samples from 3 of the 4 clinical non-responders to narsoplimab are illustrated.