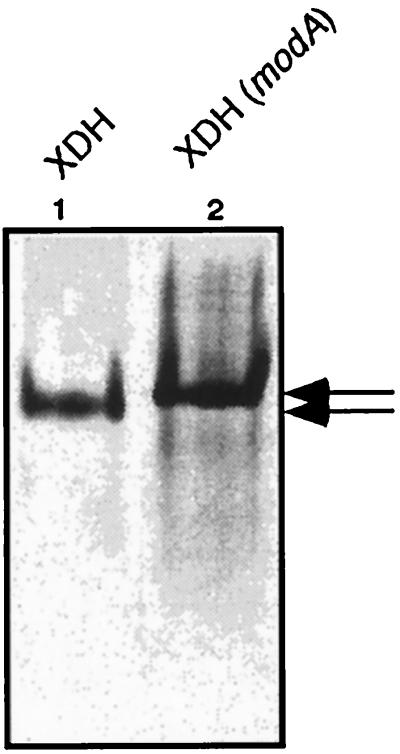
FIG. 5.
Analysis of R. capsulatus XDH isolated from the wild type and a modA mutant strain by polyacrylamide gel electrophoresis. Purified XDH was electrophoresed under nondenaturing conditions on 6% polyacrylamide gels and stained for protein with Coomassie brilliant blue. Arrows indicate different electrophoretic mobilities of active (lane 1) and inactive (lane 2) XDH. Lane 1, 3 μg of active XDH purified from R. capsulatus wild type; lane 2, 5 μg of inactive XDH purified from R. capsulatus R438I (modA), grown in the absence of molybdate.