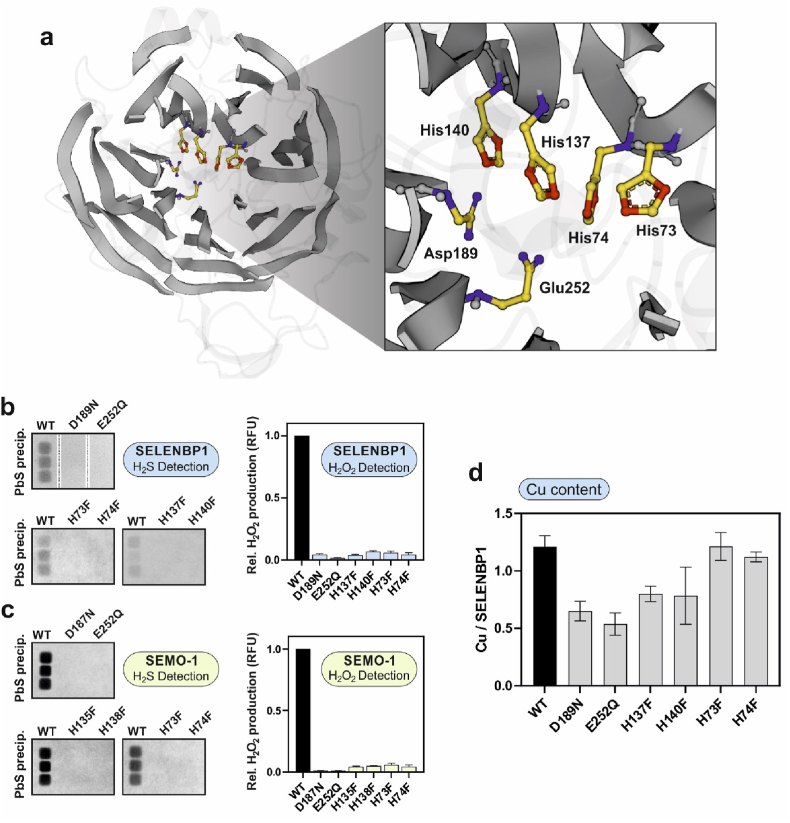
Fig. 3.
Identification of Cu-binding amino acids in human SELENBP1, and evaluation of their relevance for its MTO activity. a, 3D-model of SELENBP1 (AlphaFold), highlighting the β-propeller structure of the protein. Right panel: magnified cavity showing the modelled topological location of the amino acids proposed to mediate copper coordination (oxygen: blue; nitrogen: red; carbon: yellow). Putative Cu-binding amino acids and motifs were identified using the COFACTOR program. b, c, SELENBP1 and SEMO-1 mutants were generated through site-directed in vitro-mutagenesis. Recombinant wildtype and mutant proteins were produced in E. coli and treated with 10 μM CuCl2 during affinity purification; excess Cu2+ was removed through subsequent washing with HBS. MTO activity was tested assessing the release of H2S and H2O2 from enzymatically generated methanethiol and copper content of the proteins was determined using TXRF; three independent experiments were performed. d, Copper content of the wildtype and mutant recombinant SELENBP1 proteins. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)