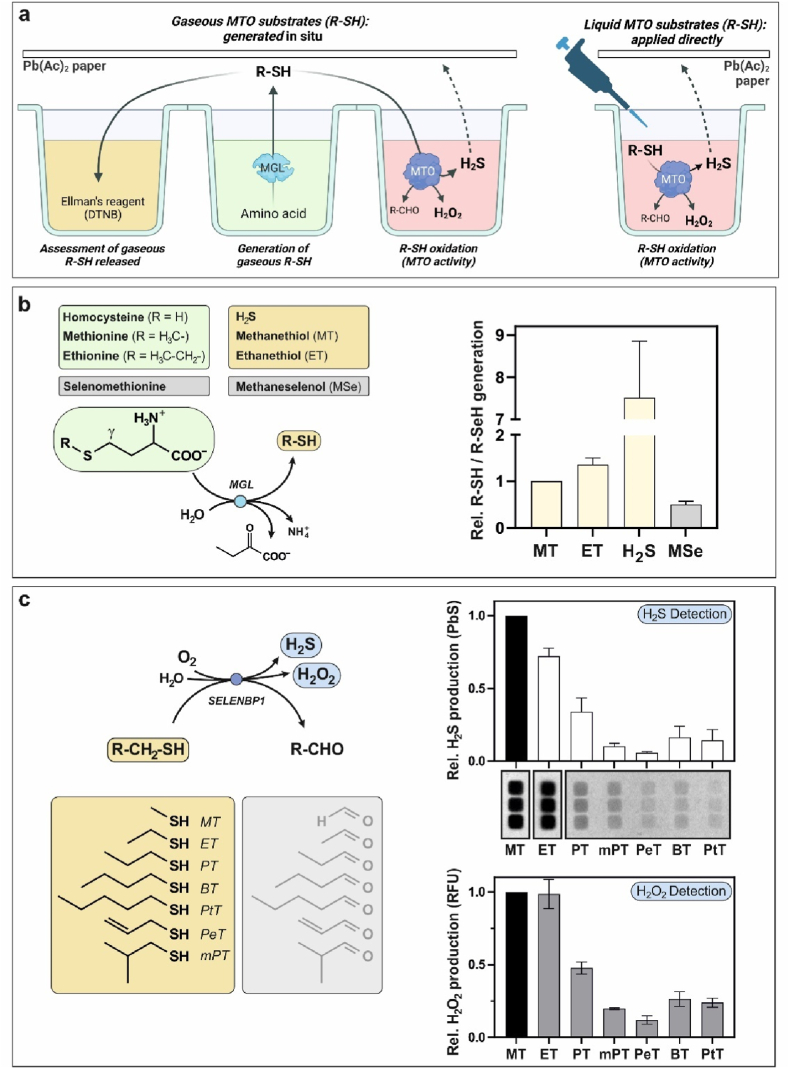
Fig. 4.
SELENBP1 substrates other than methanethiol: SELENBP1 is a thiol oxidase. a, Schematic drawing of the assay developed to test for the capability of SELENBP1 to convert different volatile and non-volatile substrates. The assay was set up in reaction wells in a 384 well-plate. Generation of potential gaseous SELENBP1 substrates through MGL-catalyzed elimination reactions was followed (in adjacent reaction wells) by assessment of the volatile thiols/selenols using Ellman's reagent with spectrophotometric measurement of TNB2− at 412 nm, and by testing for thiol oxidase activity of recombinant SELENBP1 using the volatile substrates (left panel). Liquid substrates were added directly to the SELENBP1 reaction wells (right panel). This scheme was created with BioRender.com. b, MGL-catalyzed generation of volatile thiols/selenols, as detected using Ellman's reagent. c, Detection of SELENBP1-catalyzed H2S and H2O2 production from different substrates. Substrates tested and reaction scheme are given on the left. H2S release (upper right panel) was assessed through lead (II)-acetate indicator paper; image of the lead sulfide precipitates by three independent protein isolates and densitometric quantitation of pixel density are shown. H2O2 production (lower right) was quantitated through a fluorometric HRP-coupled assay. All data are given as relative means ± SD from three independent experiments; the standard SELENBP1 substrate methanethiol was set to 1. (MT: methanethiol, ET: ethanethiol, PT: propanethiol, mPT: 2-methyl-1-propanethiol, PeT: 2-propene-1-thiol, BT: butanethiol, PtT: pentanethiol).