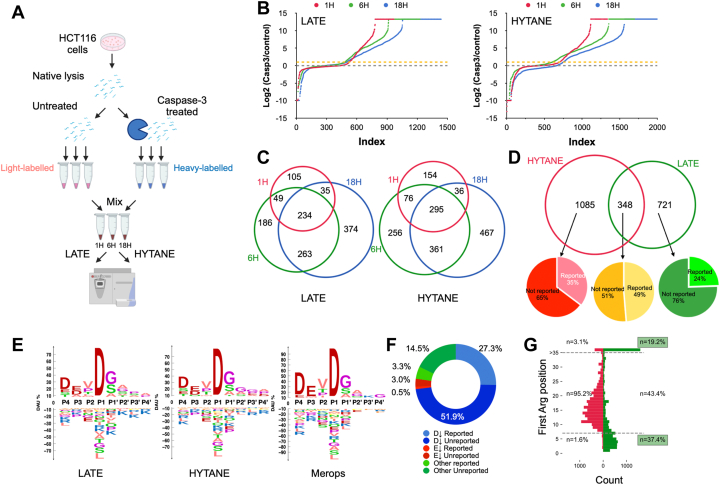
Fig. 2.
N-terminomics of caspase-3 cleavages in vitro.A, in vitro experimental scheme. The drawing was created with BioRender.com. B, ranked ratios of dimethylated peptides showing the accumulation trends of peptides with high Log2 (caspase-3/control) abundance ratio (>1 as marked by the yellow dotted line) in LATE and HYTANE. C, Venn diagram of peptides with Log2 (caspase-3/control) >1 at each time point in LATE and in HYTANE. D, Venn diagram of the unique cleavage sites after D or E that were identified only in the caspase-3-treated samples with a caspase-3/control ratio ≥2 in at least one time point. “Reported/not reported” is based on a comparison with published data. E, sequence logo of all putative cleavage sites that were identified only in the caspase-3-treated samples with a caspase-3/control ratio ≥2, in comparison with all caspase-3 cleavage found in MEROPS. F, distribution of all putative cleavage sites that were identified only in the caspase-3-treated samples with a caspase-3/control ratio ≥2 based on the amino acid at the P1 position of their cleavage motif. G, the residue distance distribution of the nearest arginine to the identified peptide sequence in LATE (green) and HYTANE (red) experiments. HYTANE, hydrophobic tagging-assisted N-termini enrichment; LATE, LysN amino terminal enrichment.