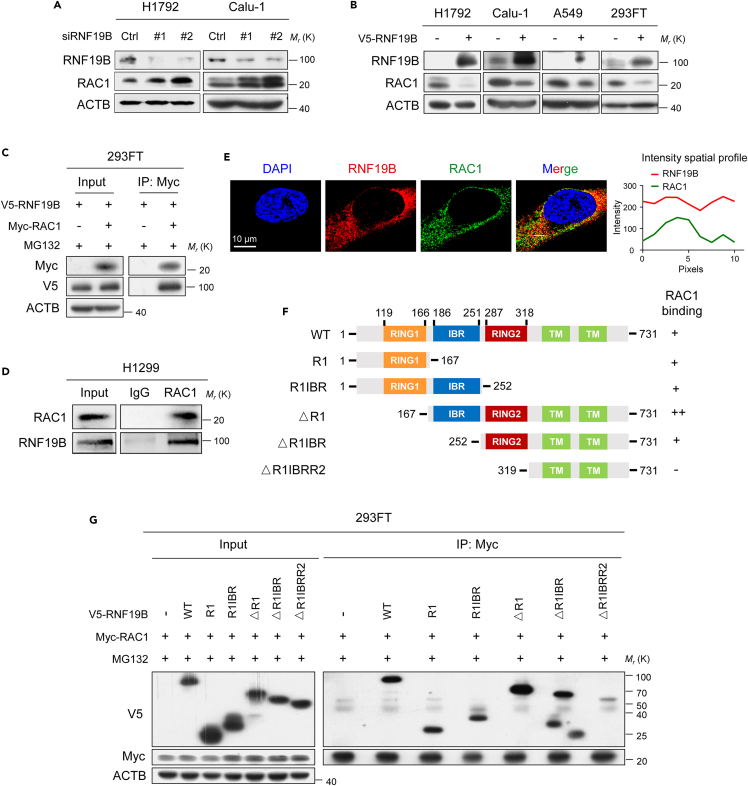
Figure 4.
RNF19B reduces the abundance of RAC1 and binds to RAC1 directly
(A) Knockdown of RNF19B expression by siRNAs in H1792 and Calu-1 cells. Cell lysates were analyzed by western blotting with antibodies against RNF19B, RAC1, and ACTB.
(B) Overexpression of RNF19B in H1792, Calu-1, A549, and 293FT cells. Cell lysates were analyzed by western blotting with antibodies against RNF19B, RAC1, and ACTB.
(C) 293FT cells were transfected with V5-RNF19B and Myc-RAC1 plasmids and were cultured for 24 h before being further incubated with MG132 (20 μM) for 6 h. Then, the co-immunoprecipitation assays were carried out with Myc antibody and the co-eluted proteins were detected by western blot assays with Myc and V5 antibodies.
(D) Co-immunoprecipitation of endogenous RAC1 with RNF19B in H1299 cells.
(E) Immunofluorescence staining for detecting endogenous RNF19B and RAC1 expression in Calu-1 cells. The intensity profiles of RNF19B and RAC1 along the white line are plotted in the right panel.
(F) The schematic representation of RNF19B domain structure and the summary of the relative binding affinity with RAC1 from 5 experimental sets.
(G) Co-immunoprecipitation analysis of the interaction between RAC1 and RNF19B or RNF19B truncation mutants in 293FT cells co-transfected with V5-RNF19B-WT plasmid or V5-RNF19B truncation mutant plasmids together with Myc-RAC1 plasmid.