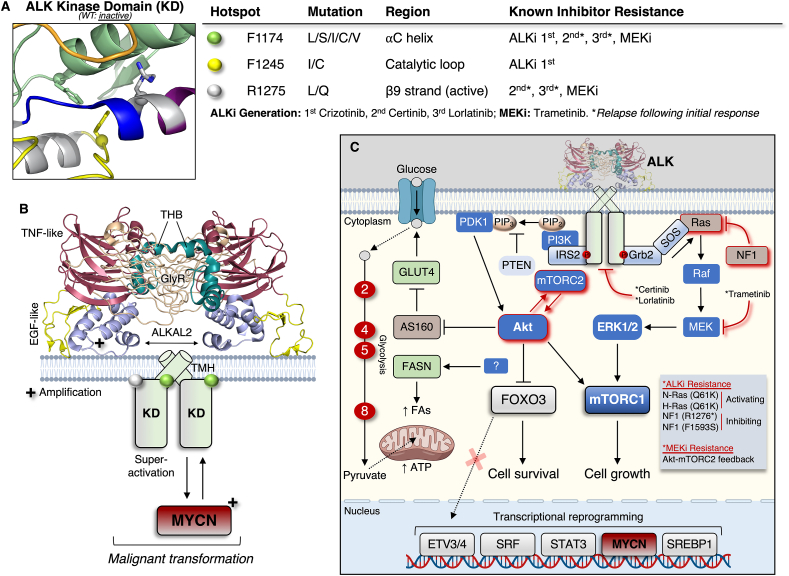
Figure 2.
ALK drug resistance and signalling in neuroblastoma. A) Three kinase domain ‘hotspot’ mutations have been identified (indicated by coloured spheres in the structure of the inactive, unliganded ALK kinase domain (PDB: 3L9P)) in neuroblastoma that are responsible for, or associated with, resistance to ALK or MEK inhibitors, resulting in MAPK and Akt signalling, respectively. An asterisk refers to cases in which ALK-mutated tumours develop resistance (e.g., N-Ras Q61K) following a period of initial positive responsiveness to the drug. Note that the R1275L/Q mutation is located where a β9-strand would normally form in the active kinase, as can be seen in Figure 1A. B) Structure (PDB: 7N00) of the dimeric, ligand-bound extracellular domain of ALK showcases several novel features of RTK including a tri-helical bundle (THB), and the glycine-rich (GlyR) and TNF- and EGF-like domains. A transmembrane helix (TMH) connects the extracellular domain to the intracellular kinase domain. In this case the more potent ALKAL2 is shown in the structure, although it should be noted that ALKAL1 is also an activatory ligand. ALKAL- and MYCN-amplification (+) are generally mutually exclusive, and ALKAL activation of mutant ALK generates a so-called super-active kinase that, in addition to mutant ALK (F1174L) alongside MYCN overexpression, promotes malignant transformation. C) Major ALK downstream signalling effectors and transcriptional modulators, and the metabolic consequences of ALK activity, including putative phospho-activation of FASN and upregulation of several enzymes in the glycolytic pathway that are numbered in red circles according to order (2: glucose-6-phosphate isomerase; 4: aldolase; 5: triosephosphate isomerase 1; 8: phosphoglycerate mutase 1). ALK and MEK drug resistance mechanisms, by way of signalling pathway activation, are indicated by red lines and described in the schematic text box.