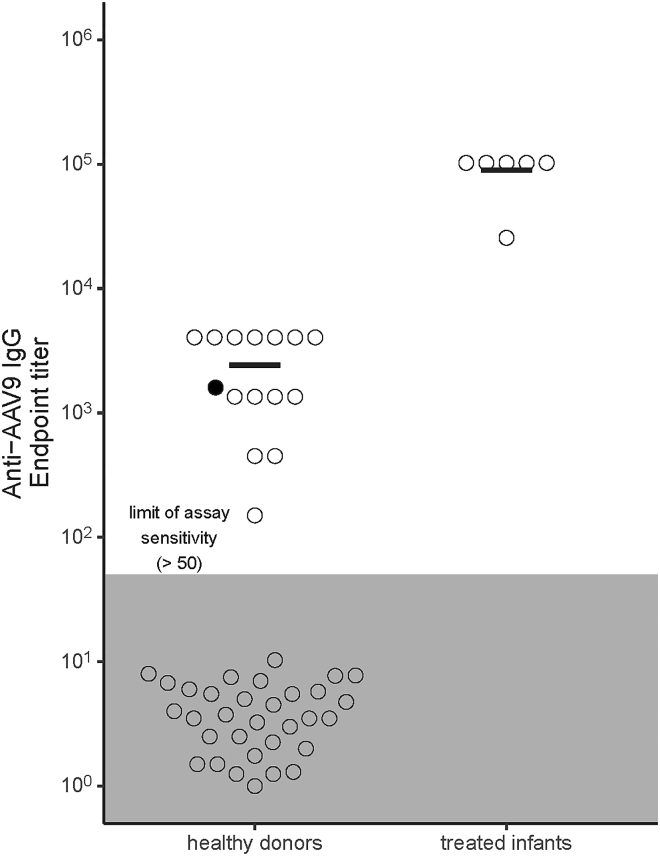
Figure 1.
AAV9-based gene therapy induces high anti-AAV9 antibody endpoint titers in treated infant sera
Sera from AAV-treated SMA infants (n = 6) and healthy donors (n = 51) were analyzed to determine anti-AAV9 IgG endpoint titers. Black bars in each group depicted in the bee swarm plot indicate the mean of samples with a detectable titer. Samples from treated infants were collected between 169 and 335 days post-treatment. The donor indicated in black is a woman whose child was excluded from access to AAV9 gene therapy because of passively acquired antibodies in utero. The gray zone indicates levels below the limit of assay sensitivity. Results are representative of three independent assays.