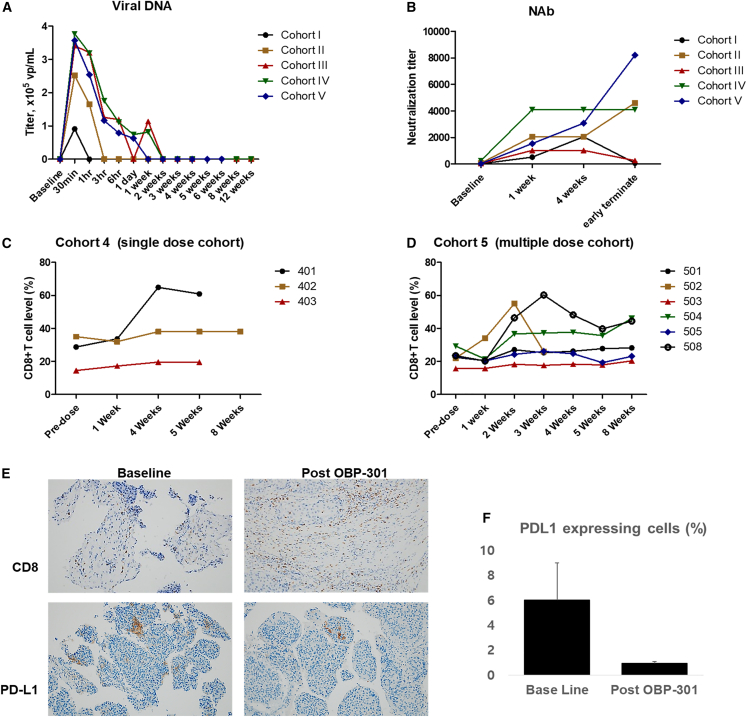
Figure 6.
Pharmacokinetics and immunological evaluations of patients at baseline and different times after OBP-301 injection(s)
(A) Quantitation of OBP-301 viral DNA in plasma samples of different cohorts at different times. Note that viral shedding was greater in patients who received higher doses. (B) Neutralizing anti-adenovirus antibody (NAb) titers in plasma samples of different cohorts at different times. Note that all patients had increased NAb titers compared with that at baseline, and there was no correlation between absolute titer and dose of OBP-301. (C) The CD8+ T cell level (%) of peripheral blood samples collected from patients in cohort 4 at different time points. (D) The CD8+ T cell level (%) of peripheral blood samples collected from patients in cohort 5 at different time points. (E) Representative immunostaining for CD8 and PD-L1. Note that OBP-301 promoted CD8+ T cell recruitment. (F) PD-L1 expressing cells (%).