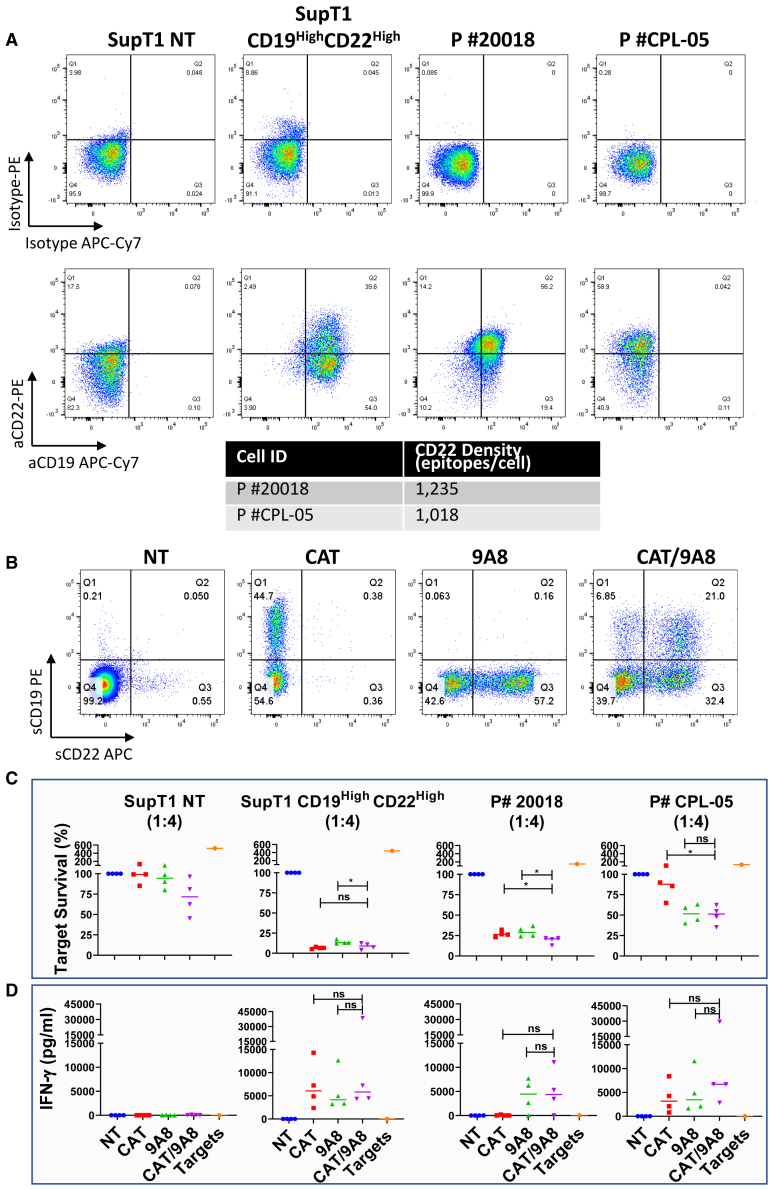
Figure 5.
Cytotoxic efficacy of CAT/9A8 against primary B-ALL patient cells
(A) The expression of CD22 and CD19 antigens was validated by flow cytometry and CD22 density was calculated by using Quantibrite beads. (B) PBMCs were transduced with either the single CAR constructs or co-transduced with the CAT/9A8 product at an MOI of 2.5 + 2.5. We validated the transduction yield by labeling the T cells with soluble antigens. (C) The cytotoxic efficacy of the CARs against the primary B-ALL samples was measured by co-culture and subsequent target cell enumeration at 48 h after co-culture (E:T = 1:4). SupT1 NT and CD19HighCD22High constituted a negative and positive lysis control, respectively. (D) IFN-γ production was measured by ELISA at 48 h, E:T ratio 1:4. Statistical significance was determined by Student's t-test (n = 4).