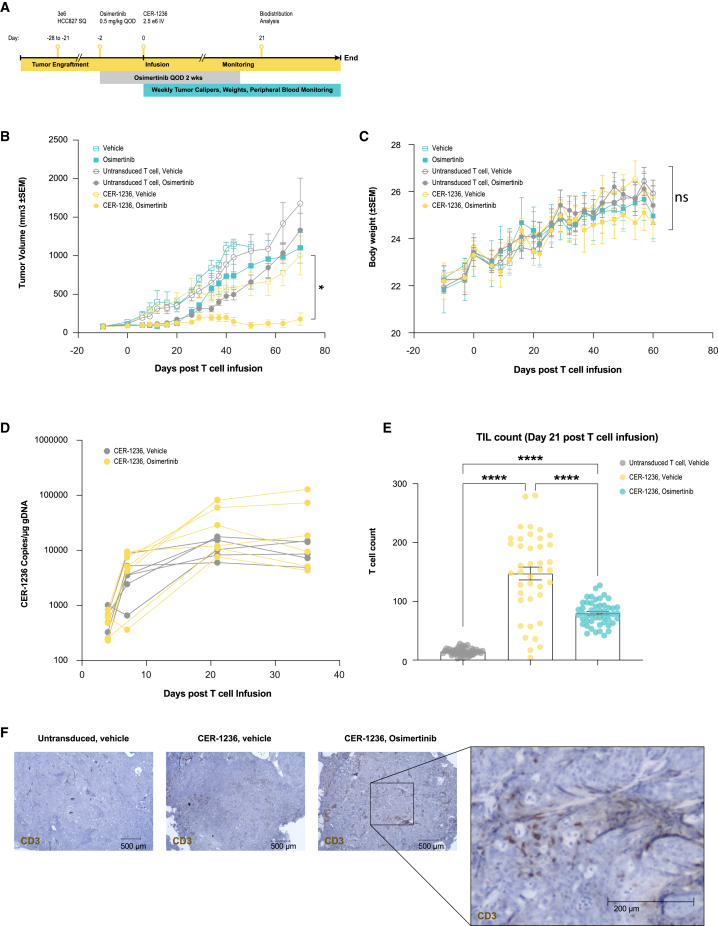
Figure 6.
CER-1236 in vivo efficacy against EGFR mutation-positive NSCLC xenografts
(A) Schematic of schedule of assessments for evaluation of CER-1236 anti-tumor activity against a localized xenografted NSCLC (HCC827) solid tumor model in NSG mice with five to eight animals per group. (B) CER-1236-treated animals led to NSCLC xenograft tumor regression, with combination of osimertinib (0.5 mg/kg, QOD for 14 days) and CER-1236 leading to the largest regression in tumor volume. Average tumor volume ± SEM. Statistics were analyzed at each time point using a mixed-effects analysis with the Geisser-Greenhouse correction with Tukey’s multiple comparisons test. Results are reported for day 70. ∗p < 0.05. (C) Osimertinib or T cell treatments did not lead to observable loss in body weight. Average body weight ± SEM. (D) CER-1236 infused to osimertinib-dosed HCC827 animals showed higher CER T cell expansion compared with CER-1236 infused in vehicle-treated controls after a single infusion of 2.5e-6 T cells. CER-1236 T cell expansion in blood was evaluated by ddPCR analysis using CER-1236-specific probes on DNA isolated from check bleed samples. Statistics were analyzed at each time point using a mixed-effects analysis with the Geisser-Greenhouse correction with Dunnett’s multiple comparisons test. (E and F) Higher T cell infiltrates were observed in CER-1236-treated animals from day 21 harvested tumor sections. The bar graph represents average CD3-stained automated T cell counts from 40 to 50 images taken at 20× magnification of two adjacent tissue sections from the same tumor from the described groups. Average ± SEM (n = 1). Statistics were analyzed using a one-way ANOVA with Tukey’s multiple comparisons test. ∗∗∗∗p < 0.0001. A 4× captured representative image with overlays of CD3 (brown)-stained sections from tumors harvested at day 21. NSCLC, non-small cell lung cancer; CER, chimeric engulfment receptor; OQD, every other day; SEM, standard error of the mean; ddPCR, digital droplet polymerase chain reaction.