Abstract
Cognitive impairments and abnormal immune activity are both associated with various clinical disorders. The association between C-Reactive protein (CRP), a marker associated with inflammation, and cognitive performance remains unclear. Further, mechanisms potentially linking CRP to cognition are not yet established. Brain structure may well mediate this relationship: immune processes play crucial roles in shaping and maintaining brain structure, with brain structure and function driving cognition. The United Kingdom Biobank (UKBB) is a large cohort study with extensive assessments, including high-sensitivity serum CRP levels, brain imaging, and various cognitive tasks. With data from 39,200 UKBB participants, we aimed first to determine the relationship between CRP and cognitive performance, and second, to assess metrics of brain morphology as potential mediators in this relationship. Participants were aged 40 to 70 at initial assessment and were mostly Caucasian. After accounting for potential covariates (e.g., age, sex, medical diagnoses, use of selective-serotonin reuptake inhibitors), we found CRP levels to have small, negative associations with fluid intelligence (b = −0.03, 95%CI[-0.05,-0.02], t(14381) = −3.62, pcor = .004), and numeric memory (b = −0.03, 95%CI[-0.05,-0.01], t(14366) = −3.31, pcor = .007). We found no evidence of brain morphology mediating these relationships (all |ab| < 0.001, all pcor > .55). Our findings from this large sample suggest that serum-assessed CRP is of marginal importance for cognitive performance in mid-to-late aged Caucasians; the small effect sizes of statistically significant associations provide context to previous inconsistent results. The seeming lack of involvement of brain morphology suggests that other brain metrics (e.g., connectivity, functional activation) may be more pertinent to this relationship. Future work should also consider CRP levels measured in the central nervous system and/or other cytokines that may better predict cognitive performance in this population.
Keywords: Inflammation, Fluid intelligence, Numeric memory, United Kingdom biobank, Volume, Thickness, Surface area, FreeSurfer
Highlights
-
•
CRP was marginally associated with scores of numeric memory and fluid intelligence.
-
•
No evidence that brain morphology mediated these relationships.
-
•
Brain function and/or connectivity better mediate the link between CRP and cognition.
-
•
Other cytokines associated with inflammation maybe more relevant for cognition.
1. Introduction
Reduced cognitive performance (e.g., in memory, attention, executive function) and abnormal immune function (e.g., inflammation) co-exist across many disorders, including various psychiatric conditions (Millan et al., 2012; Bauer and Teixeira, 2019; Fourrier et al., 2019), neurological conditions (Lai et al., 2017), and other medical conditions like obesity, and diabetes (van den Berg et al., 2009; Gregor and Hotamisligil, 2011). A common marker of immune activity, C-reactive protein (CRP) is known as an acute-phase protein and is associated with inflammatory processes (Gabay and Kushner, 1999; Pepys & Hirschfield, 2003). While most individuals have CRP levels around or below 1 mg/L, CRP levels can increase drastically under various conditions including times of infection (Pepys, Hirschfield). Various clinical populations are associated with chronic CRP levels above 3 mg/L (Pepys, Hirschfield; Pearson et al., 2003).
Literature regarding the association between CRP and cognitive performance is inconsistent. After controlling for established confounding factors (e.g., age, sex, ethnicity, medical diagnoses, medication use; reviewed in (O'Connor et al., 2009)), studies on non-clinical middle-to-late aged human populations (generally 50 years and older) have found associations between CRP and: memory (particularly verbal memory (Gimeno et al., 2008; Noble et al., 2010; Bettcher et al., 2012; Marsland et al., 2015)), spatial reasoning (Marsland et al., 2015), attention (Beydoun et al., 2018), executive function (Schram et al., 2007; Wersching et al., 2010; Tegeler et al., 2016), and measures of global cognition (Schram et al., 2007; Yaffe et al., 2003). However, findings from other similar samples do not find evidence of these associations between CRP and memory (Beydoun et al., 2018; Wersching et al., 2010; Tegeler et al., 2016; Dik et al., 2005; Weuve et al., 2006; Jordanova et al., 2007; Rafnsson et al., 2007; Luciano et al., 2009; Tampubolon, 2016), spatial reasoning (Luciano et al., 2009), executive function (Noble et al., 2010; Beydoun et al., 2018), nor global cognition (Dik et al., 2005; Weuve et al., 2006; Jordanova et al., 2007). Though it is unclear what explains these varying results, different methods in profiling CRP (e.g., aggregating CRP measures with measures of other inflammation-associated-cytokines, reviewed in (Walker et al., 2022)), different cognitive tests (e.g., global versus domain-specific cognition versus cognitive screening measures), and differences in using these test scores (e.g., classifying participants as being above or below a cognitive impairment threshold score) as well as sample characteristics may all play a role.
Insofar as CRP levels and cognitive performance are associated, mechanisms that may link inflammation and cognitive performance remain unclear. Brain morphology is a possible mediator in this relationship: brain morphology is reliably associated with cognitive abilities (Cox et al., 2019; Khalil et al., 2022) as well as CRP-levels (Frodl and Amico, 2014). Short-term learning and memory are associated with prefrontal, parietal, temporal, and more ambiguously, cerebellar brain regions with longer term consolidation also involving the medial temporal lobe (Zimmerman et al., 2006; Kaup et al., 2011; Brem et al., 2013). Higher-level cognitive processes, including intelligence and executive functioning, have been associated with total brain volume, insula volume, and temporal lobe volume (Cox et al., 2019; Zimmerman et al., 2006). Similarly, increased CRP-levels have been associated with decreases in whole brain volume (Jefferson et al., 2007; Satizabal et al., 2012), volume and surface area of overall grey matter (Marsland et al., 2015; Satizabal et al., 2012; Gu et al., 2017), volumes of the temporal lobe including structures like the hippocampus (Marsland et al., 2015; Frodl and Amico, 2014; Satizabal et al., 2012; ; ; Taki et al., 2013; Warren et al., 2018; ), as well as increased white matter hyperintensities (Wersching et al., 2010). As with CRP-cognition associations, studies relating CRP and metrics of brain structure have used various methods for measuring CRP and brain structure, for accounting for confounding variables, and for statistical analyses. These methodological differences as well as use of various samples (often focused on clinical and older-aged populations) may explain why specific relationships between CRP and brain structure remain unclear. Nonetheless, some recent studies investigated CRP-cognition associations with mediations through brain structure with middle-to-late aged non-clinical samples. Marsland et al. (2015) reports results on mediation analyses between CRP, brain morphology and cognitive performance in a sample of a middle-aged participants. They found that overall grey matter volume partially mediates associations between CRP and spatial reasoning, short-term memory, verbal learning and memory, and executive function. Work by Bettcher et al. (2012) on older adults found CRP to associate with both decreased left medial temporal lobe volume and worsened performance on verbal memory tasks, indicating a potential mediation effect, though a formal mediation model was not tested. A recent investigation in older adults found few associations between serum CRP and brain morphology but stronger associations, particularly to measures of white matter when considering epigenetic markers of CRP expression (Conole et al., 2021). However, a recent analysis on data from the United Kingdom Biobank (UKBB) failed to find associations between CRP (when considering both serum concentrations and genetic markers) and brain morphology when controlling for multiple comparisons; with results unchanged when excluding clinical participants (Williams et al., 2022). These inconsistent results reflect the lack of clarity in associations between CRP and brain structure (including if CRP affects certain brain structures more than others) and how such CRP-brain associations may affect cognitive performance (generally and in terms of specific cognitive domains). Moreover, as many of these studies are observational, there are few indications about the directionality of these potential associations. Finally, as literature on this question tends to focus on older-adults as well as on clinical populations, little is known about CRP-brain-cognition associations over life course.
The UKBB is a prospective cohort study in the United Kingdom that, since 2006, has recruited over 500,000 participants aged 40 to 73 (https://www.ukbiobank.ac.uk/). With comprehensive data at multiple time points on various biological, cognitive, and lifestyle variables, the UKBB's well characterized sample provides an exciting opportunity to address limitations in previous works and to replicate previous associations between CRP, cognitive performance, and brain morphology. In particular, with the UKBB's large sample, we aimed to clarify the association between CRP and specific cognitive domains, while rigorously accounting for the numerous factors known to confound these associations. Further, using a priori mediation models, we aimed to determine the potential mediating role of general and specific brain morphology measures taken from magnetic resonance imaging (MRI) in CRP-cognition associations. These mediation models and the temporal relationship of their variables may help establish directionality in associations between CRP and cognition. Finally, through various supplementary analyses, this work may well provide insights to how life course and neuropsychiatric diagnoses may affect CRP-brain-cognition associations.
2. Material and methods
2.1. UK biobank – participants & design
All UKBB participants completed assessments related to health and lifestyle at a baseline assessment (2006–2010, referred to by UKBB as instance 0). Participants were then randomly selected to participate in follow-up assessments. Follow-up 1 assessments (UKBB: instance 1) were conducted from 2012 to 2013 and follow-up 2 (UKBB: instance 2) began in 2014. Follow-up 2 assessments included MRI scans for 39,200 participants. Informed consent was collected from all participants used in these analyses; for details on UKBB ethics frameworks and procedures, see https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/about-us/ethics. Data from this project are accessible to researchers upon approval, and procedural documentation are publicly available on the UKBB website.
Authors DM, ML, and KML, received the requisite approval to access and analyse UKBB data for this project as part of a larger application through McGill University (PI: Alan C. Evans, Application Number 45551); only these authors accessed raw data. The UKBB data was hosted on servers maintained by Calcul Québec in association with the Digital Research Alliance of Canada (https://alliancecan.ca/en) and managed through NeuroHub, an infrastructure funded in part by the Canada First Research Excellence Fund, awarded through the Healthy Brains, Healthy Lives initiative at McGill University.
2.2. Procedures & measures
Given the availability of data and to maintain temporal precedence, this current study included blood samples from the baseline assessment along with metrics of brain morphology and cognitive performance at follow-up 2; the average delay between baseline assessments and follow-up 2 assessments was 9.6 years (SD = 1.2). Potential confounding variables were taken at both baseline and follow-up 2 assessments. Supplementary table A1 provides a complete list of variables used in the current project and their UKBB reference field code.
2.2.1. CRP
Blood samples were collected at the baseline assessment, between 17th April 2007 and 30th October 2010 for the participants whose results are presented in this manuscript. CRP levels were measured between 1st November 2015 and 3rd October 2017 using independently validated high sensitivity-CRP immunoturbidimetry assays sensitive to CRP concentrations between 0.08 and 80 mg/L (Beckman Coulter, UK) (Fry et al., 2019). CRP data from the baseline assessment was used for all analyses as this allowed for the largest sample size (29,000 participants had CRP data at baseline and neuroimaging data at follow-up 2, compared to n = 2034 participants who had CRP data at follow-up 1). At the time of writing, the UKBB did not have accessible CRP data at follow-up 2.
2.2.2. Brain morphology
MRI scans were conducted using a Siemens Skyra 3T scanner with VD134 SP4 software and a 32-channel RF receiver head coil with previously described procedures (Smith et al., 2020). Briefly, T1 weighted, and T2-weighted FLAIR MRI were performed. T1-weighted images were acquired over 5 min and used a 3D MPRAGE sequence: 1 mm3 resolution, repetition time (TR) = 735ms, echo time (TE) = 39ms, in plane acceleration (iPAT) = 2, with the prescan normalize filter applied. T2-weighted FLAIR images were acquired over 6 min and used a 3D SPACE sequence: 1.05x1x1mm resolution, TR = 5000ms, TE = 395ms, iPAT = 2, partial Fourier = 7/8, along with fat saturation, elliptical k-space scanning and the prescan normalize filter.
Images were processed through the FreeSurfer pipeline (version 6.0; https://surfer.nmr.mgh.harvard.edu/) (Fischl, 2012). FreeSurfer uses T1 and T2-FLAIR images to compute the surface area, mean thickness, and volume of brain regions defined by the Desikan-Killiany-Tourville (DKT) atlas for cortical regions and the Automatic Subcortical Segmentation (ASEG) atlas for subcortical regions (Fischl, 2012; Dale et al., 1999; Klein and Tourville, 2012). FreeSurfer's computations were quality-controlled, as described previously (Alfaro-Almagro et al., 2018).
2.2.3. Cognition
Cognitive performance was assessed on the same day as MRI scans. The cognitive measures used by the UKBB show moderate to high correlations with appropriate reference tests and acceptable test-retest validity (for details on reliability, see (Fawns-Ritchie and Deary, 2020)). The cognitive tasks administered are described below in order of administration (see the UKBB website and (Fawns-Ritchie and Deary, 2020) for additional details).
To measure reaction time, participants were shown two cards at a time and instructed to press a button as quickly as possible if both cards were identical. Twelve trials were conducted with the final metric being the mean time between the cards’ presentation and the button press.
The symbol-digit substitution task was also administered. In this task, participants were provided a sequence of symbols to decode from a legend that provided a corresponding digit for each symbol. The number of correct matches made in a 1-min period was of interest.
The fluid intelligence task consisted of 13 multiple choice questions. Each question was related to verbal or numeric reasoning. The fluid intelligence score is the number of correct answers in a 2-min period.
The numeric memory or digit span test was used to assess working memory. In this task, participants were shown a number, at first with two digits, and asked to recall this number after a 3 s delay. After each correct recall, one digit was added to the number, up to a maximum of 12 digits. The test ended after two successive incorrect answers if the number was three or more digits long or after five successive incorrect answers for two-digit numbers. The maximum number of digits successfully remembered was used.
The Trail Making Test (TMT) was also administered. In this task, participants were shown a set of numbers (numeric) or letters and numbers (alphanumeric) scattered around the screen. Participant were instructed to click on these elements sequentially. For each of these tests, two metrics were of interest: the time to complete the tests (duration) and the number of errors made while completing the test.
In the Tower Rearrangement Test, participants were shown three pegs with three coloured hoops. Participants were instructed to indicate the number of moves necessary to match the illustration to a target illustration showing the hoops in a different arrangement. The number of puzzles correctly solved in 3 min was used and is thought to reflect executive function.
The Matrix Pattern Completion Task consisted of a matrix of elements with a pattern of characteristics. Each matrix was missing one element, participants were instructed to choose the element that completed this matrix from a bank of possible answers. The number of matrices correctly completed in 3 min was used.
Note that the UKBB's raw cognition data included values indicating when a participant did not complete or abandoned a cognitive assessment; these values were set to missing in our analyses.
2.2.4. Potential covariates
Potential confounding factors were identified from previous literature (O'Connor et al., 2009) and included age, sex, social economic status, ethnicity, waist-to-hip ratio, activity level, current smoking status, alcohol consumption, sleep duration (self-reported average), medical diagnosis, and use of SSRI, antihypertensive and statin medications. Further given our interest in brain morphology, head size and handedness were included as covariates (Sommer et al., 2001). As most of the sample was of Caucasian ethnicity, “British”, “Irish”, “White” and “Any other white background” were coded as “White”, while all other categories were grouped into “Non-white”. Social economic status was measured by the Townsend deprivation index (Townsend, 1987). This scale summarises deprivation across thirteen areas of life, including diet, clothing, social activities, and work, among others. In the UKBB, a score on the Townsend deprivation index was calculated based on census data based on the participants' postal code. Activity level was measured by the International Physical Activity Questionnaire's (IPAQ) (Hagstromer et al., 2006). Participant's IPAQ responses were categorized into high (approximately 1 h or more of moderate and/or half an hour of vigorous physical exercise per day), moderate (approximately half an hour of moderate-intensity physical exercise on most days) or low (neither high nor moderate). The time of day of the blood draw, fasting time, and delay between blood draw and CRP measurement were not considered as potential confounding factors as they were not associated with the log-transformed CRP values (Spearman's |r|≤.01, p ≥ .07); this is consistent with established findings (Pepys and Hirschfield, 2003).
Given known associations between CRP and certain medical diagnoses and SSRI use, these variables were used as exclusion criteria in the main analyses (O'Connor et al., 2009). These were controlled for by exclusion rather than statistically for three reasons: (1) given the relatively few such cases in the sample, (2) to reduce potential for multi-collinearity in the statistical models, and (3) in an effort to ease comparisons with other related studies that have done similarly (e.g., Marsland et al., 2015). Relevant diagnoses included mood disorders, schizophrenia spectrum disorders, dementia, sleep disorders, alcohol dependence, cardiovascular disease and current infections. For transparency, the use of aspirins, immunosuppressants, and antipsychotics medications are described given potential associations with CRP (O'Connor et al., 2009). For specific values determined to fit these diagnosis and medication categories, see supplementary tables B1 and B2.
Instead of including BMI as a covariate, waist-to-hip ratio was chosen given suggestions that waist-to-hip ratio is more strongly associated with changes in CRP than BMI (O'Connor et al., 2009). As including both BMI and waist-to-hip ratios in the statistical models described below, may have introduced multi-collinearity to the model, waist-to-hip ratio was retained in the statistical models. Variables that served as exclusion criteria, namely diagnosis of certain disorders and SSRI use, are described. All measures described in this section were collected via computerized surveys.
2.3. Statistical analyses
Participants missing CRP values at baseline, missing MRI values at follow-up 2, or missing values on at least one covariate at baseline or follow-up 2 were excluded from analyses (missing data: n = 20,195). Participants with data for at least one cognitive task were retained and included in analyses when possible. The number of missing values per variable are detailed in Supplementary table C1. Though no such cases remained after removing missing values, participants with CRP values above 10 mg/L would have been excluded, given this study's interest in low-level chronically elevated CRP levels (Pearson et al., 2003).
The complete cases (n = 19,005) were divided into subsets based on theoretical considerations including diagnosis of a disorder associated with CRP-levels and cognitive performance, use of medications with similar associations, and age (see Supplementary table D1 for details on each of the eleven subsets). Importantly, this manuscript focuses on results from the subset that excluded participants with various neuropsychiatric diagnosis and use of SSRIs at either baseline or follow-up 2, like previous studies on similar questions (O'Connor et al., 2009; Marsland et al., 2015). To determine the potential effects of removing these cases, the retained cases were compared to the excluded cases on demographic variables (age, sex, ethnicity, social economic status), as well as CRP and other variables previously associated with the primary variables of interest (BMI, waist-to-hip ratio, sleep, alcohol consumption, medication use, activity level, smoking status, menopause, dominant hand) (O'Connor et al., 2009; Sommer et al., 2001). The statistics for these comparisons included t-test statistics for normally distributed numeric variables, Mann-Whitney-Wilcoxon test statistics for non-normally distributed continuous variables, and chi-square statistics for categorical variables. Hedge's g was calculated for comparisons on normal continuous variables, r for non-normal continuous variables, and Cramer's v for categorical variables (Durlak, 2009).
First, correlations between log CRP levels and performance on cognitive tasks were conducted. Then, cognitive tasks associated with log CRP levels were entered into mediation models with log CRP levels as the predictor, brain morphology variables (volume, surface area, thickness) as the mediator, and cognitive performance variables as the outcome variable. We performed two sets of mediation analyses: first on total and lobar (frontal, temporal, parietal, occipital) brain metrics and second on regional brain metrics (see supplementary table E.1 for specific brain variables used in each round of mediation analyses). To compute lobar values, DKT regions were grouped as in Klein & Tourville (Klein and Tourville, 2012) while attributing rostral and caudal anterior cingulate cortex to the frontal lobe, and the isthmus and posterior cingulate to the parietal lobe (following Marsland et al., 2015). Lobar volume and surface area were computed by summing these measures across the lobe's respective DKT regions. Mean thickness of the lobes was taken to be the average value across the lobe's respective DKT regions.
In all mediation models, CRP values were log-transformed given its skewed distribution (Pearson et al., 2003). Linear models for the direct effect, a-path, and b-path included the following covariates: sex, ethnicity, Townsend score, age at baseline, days between baseline and follow-up 2 assessments, mean waist-to-hip ratio from baseline and follow-up 2, mean sleep duration from baseline and follow-up 2, IPAQ activity level, smoking status at baseline, smoking status at follow-up 2, use of antihypertensive medication at either baseline or follow-up 2, use of statins medications at either baseline or follow-up 2, as well as head size and dominant hand (given the latter two variables' association with brain morphology (Dale et al., 1999; Sommer et al., 2001)). Note that in the supplementary analyses on the NoMed and NoDxNoMed subsets, antihypertensive and statin medication use were not included as covariates as they were exclusion criteria for the subsets. To control for multi-collinearity, added-variable plots were produced and visually inspected to ensure that each of these covariates explained unique variance in general cognitive performance (see supplementary figure F.1). Further, standardized root mean square residuals (SRMR) were computed to evaluate models’ fit. To control for multiple comparisons, p-values were corrected using the false discovery rate (FDR) procedure with an α = .05 (Benjamini and Hochberg, 1995); herein, pcor denotes FDR corrected p-values.
To investigate the stability of CRP across time, a preliminary correlational analysis was computed on the 6931 UKBB participants with CRP data from baseline and follow-up 1. Blood draws from these assessments were, on average, 4.3 years apart (SD = 0.93). These repeated measures were moderately correlated (Spearman's r = 0.63, p < .001).
Statistical analyses were performed on a Windows 10 64-bit system in Rstudio 2023.03.1 (Build 446) running R version 4.1.1. Mediation analyses were conducted with the lavaan package (version 0.6–15). All R packages used are detailed in supplementary table G.1. Scripts for those analyses, and the python 3.8.10 scripts used to retrieve the data from host servers are publicly available (see https://github.com/mendelsonD/immunoCognition-UKBioBank, https://github.com/mendelsonD/CognitiveSubtypes/tree/main/src/create_dataset, respectively).
3. Results
Of the 39,200 UKBB participants completing follow-up 2, 19,005 had complete data on the variables of interest (see section 2.3). After excluding cases with a neuropsychiatric diagnosis (n = 3,943) and then cases using SSRIs at baseline or follow-up 2 (n = 550), 14,512 participants remained. Table 1 compares the characteristics of participants retained for these analyses to those of excluded participants. Retained participants were between 40 and 70 years old at baseline, were roughly half female and were mostly White. The retained and excluded cases were similar though the excluded cases tended to be younger at baseline (g = 0.21), and to have a small increase in CRP (r = 0.11). See supplementary data (“PartChars”) for characteristics of other subsets.
Table 1.
Participant characteristics and comparison to excluded cases.
| Total |
Retained |
Excluded |
||
|---|---|---|---|---|
| (n = 39,200) | (n = 14,512) | (n = 24,688) | ES | |
| Age at baseline (M, SD) | 54.82 (7.45) | 53.85 (7.32) | 55.38 (7.48) | g = 0.21 |
| Age at fup 2 (M, SD) | 63.63 (7.54) | 63.50 (7.38) | 63.70 (7.63) | g = 0.03 |
| Sex (% Female) | 52.90% | 51.40% | 53.70% | v <.001 |
| Ethnicity/Race (% White) | 97.00% | 97.30% | 96.80% | v <.01 |
| Townsend score (Mdn, IQR)1 | −2.60 (3.39) | −2.68 (3.30) | −2.56 (3.45) | r = .02 |
| Waist-to-hip at baseline (M, SD) | 0.86 (0.09) | 0.85 (0.08) | 0.86 (0.09) | g = 0.10 |
| BMI at baseline (kg/m2; Mdn, IQR)1 | 25.99 (5.17) | 25.53 (4.76) | 26.29 (5.41) | r = .10 |
| Waist-to-hip at fup 2 (M, SD) | 0.87 (0.09) | 0.87 (0.09) | 0.87 (0.09) | g = 0.002 |
| BMI at fup 2 (kg/m2, Mdn, IQR)1 | 25.87 (5.31) | 25.38 (4.94) | 26.23 (5.55) | r = .11 |
| Avg sleep hours at baseline (Mdn, IQR) 1,a | 7.00 (1.00) | 7.00 (1.00) | 7.00 (1.00) | r = .01 |
| Avg sleep hours at fup 2 (Mdn, IQR) 1,a | 7.00 (1.00) | 7.00 (1.00) | 7.00 (1.00) | r <.001 |
| Alcohol intake day before baseline (Mdn, IQR) 1 | 3.85 (24.00) | 7.05 (24.94) | 0.14 (24.00) | r = −.01 |
| Alcohol intake day before fup 2 (Mdn, IQR) 1 | 7.05 (28.25) | 7.19 (30.29) | 7.05 (27.97) | r <.001 |
| CRP (mg/L; Mdn, IQR) 1 | 1.07 (1.62) | 0.93 (1.31) | 1.18 (1.84) | r = .11 |
| Log CRP (M, SD) | 0.13 (1.02) | −0.04 (0.92) | 0.23 (1.07) | g = 0.27 |
| Any diagnosis (% Yes) b | 23.60% | – | 37.80% | v <.01 |
| Dementia diagnosis (% Yes) | 0.30% | – | 0.40% | v <.01 |
| SSD diagnosis (% Yes) | 0.10% | – | 0.20% | v <.01 |
| Mood disorder diagnosis (% Yes) | 3.50% | – | 5.60% | v <.01 |
| Any medication at baseline (% Yes) c | 21.30% | 15.00% | 24.90% | v <.01 |
| SSRI use at baseline (% Yes) | 2.90% | – | 4.60% | v <.01 |
| Any medication at fup 2 (% Yes) c | 28.70% | 22.50% | 32.30% | v <.01 |
| SSRI use at fup 2 (% Yes) | 3.70% | – | 5.80% | v <.01 |
| IPAQ activity level | v <.01 | |||
| Low | 18.60% | 17.80% | 19.10% | |
| Moderate | 41.90% | 42.80% | 41.20% | |
| High | 39.60% | 39.40% | 39.70% | |
| Current smoker at baseline (% Yes) | 6.30% | 5.50% | 6.70% | v <.01 |
| Current smoker at fup 2 (% Yes) | 4.10% | 2.80% | 4.90% | v <.01 |
| Menopause at baseline d | v <.01 | |||
| Yes | 53.90% | 51.10% | 55.40% | |
| No | 30.90% | 35.70% | 28.20% | |
| Not sure - had a hysterectomy | 10.10% | 7.70% | 11.40% | |
| Not sure - other reason | 5.20% | 5.40% | 5.00% | |
| Menopause at fup 2 d | v <.01 | |||
| Yes | 81.00% | 84.10% | 79.20% | |
| No | 5.40% | 4.70% | 5.80% | |
| Not sure - had a hysterectomy | 9.80% | 7.50% | 11.10% | |
| Not sure - other reason | 3.80% | 3.60% | 3.90% | |
| Dominant hand | v <.01 | |||
| Right | 89.10% | 88.90% | 89.20% | |
| Left | 9.40% | 9.70% | 9.20% | |
| Both | 1.60% | 1.40% | 1.60% |
Note. fup 2 – follow-up 2; BMI – body mass index; CRP – C-reactive protein blood aliquot concentration; SSD – schizophrenia spectrum disorder; SSRI – selective serotonin reuptake inhibitor; IPAQ – International Physical Activity Questionnaire; BMI – body mass index. 1non-normal variable; aAverage hours of sleep per night (self-reported); bAny diagnosis of interest described above; cAny medication of interest described above; dMenopause status applies only to women.
3.1. Association between CRP and cognitive variables
After controlling for potentially confounding factors and after applying FDR correction, log CRP was associated with fluid intelligence score (b = −0.03, 95%CI[-0.05,-0.02], t(14381) = −3.62, pcor = .004), and the numeric memory (digit span) task (b = −0.03, 95%CI[-0.05,-0.01], t(14366) = −3.31, pcor = .007); all other cognitive variables had |b| ≤ 0.02 and pcor ≥ .18 (appendix H details results of CRP's association with other cognitive tasks in this subset and others). In other words, a one standard deviation increase in the log of CRP was associated with a 0.03 standard deviation decrease in the UKBB's measure of fluid intelligence and similarly for the numeric memory task. For comparison, a one standard deviation increase in age at baseline was associated with a 0.07 standard deviation decrease in fluid intelligence (b = −0.07, 95%CI[-0.09, −0.06], t(14381) = −8.83, p < .001). Thus, the effect of a one standard deviation increase in the log of CRP (roughly equivalent to an increase of 8.3 mg/L) on performance on the fluid intelligence task was equivalent to the effect of a 3.4-year increase in age at baseline. Likewise, numeric memory was negatively associated with age at baseline (b = −0.12, 95%CI[-0.14, −0.11], t(14366) = −14.47, p < .001). The effect of a one standard deviation increase in the log of CRP on numeric memory was equivalent to the effect of a 1.9-year increase in age at baseline.
3.2. Mediation with brain metrics
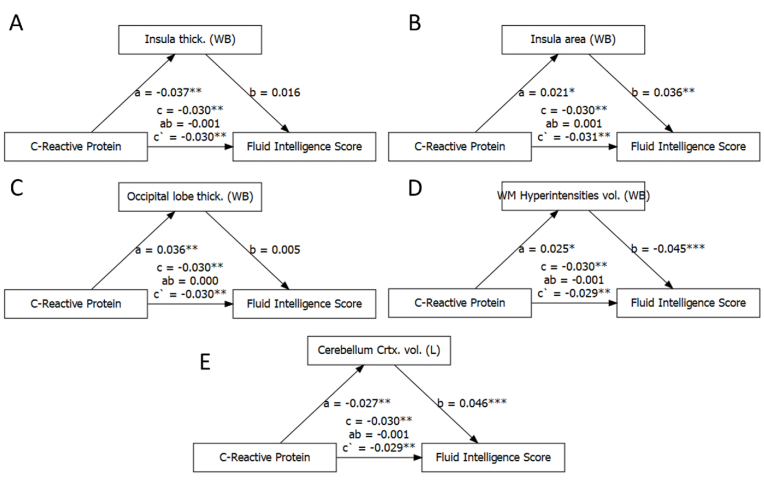
Mediation analyses were conducted on fluid intelligence and numeric memory. First, total and lobar brain metrics were included in these mediation models. CRP was associated with certain general brain metrics, including whole brain insula mean thickness and surface area, whole brain occipital lobe mean thickness, whole brain volume of white matter hyperintensities, and left cerebellum cortex volume (Fig. 1; see supplementary figures in Appendix I). CRP was not associated with total brain volume nor lobar brain volumes (both |b| < 0.009, with pcor > .28). Importantly, none of the general brain metrics mediated the relationship between log CRP and fluid intelligence nor log CRP and numeric memory (|ab| < 0.001, all pcor > .56, all |percent mediated| < 4.0%).
Fig. 1.
Mediation analysis between CRP, general brain metrics and fluid intelligence.
Note. A) whole brain insula thickness, B) whole brain insula surface area, C) whole brain occipital lobe thickness; D) white matter hyperintensities volume; E) left cerebellum cortex volume; * pcor <.05, ** pcor <.01, *** pcor <.001.
Then, to assess possible regional effects of CRP, mediation models were computed using the mean thickness, surface area, and volume of specific brain regions as mediators. These analyses found no evidence that regions defined in the DKT and ASEG atlases mediate the relationship between CRP and fluid intelligence nor numeric memory (pcor > .99; all |percent mediated| < 3.6%). As Fig. 2 illustrates, indirect effect estimates for models including DKT and ASEG regions ranged from ab = −0.0009 to ab = 0.001 (details available in supplementary data, “medResults”). The supplementary analyses reveal qualitatively similar results for other subsets (see supplementary data “MedResults”). SRMR results indicated excellent model fit for every mediation model (all SRMR <0.001; see supplementary data “MedFit”).
Fig. 2.
Results of specific brain measures mediating the association between CRP and fluid intelligence and numeric memory.
Note. Estimate of the indirect effect of log CRP on A) Fluid intelligence score and b) Numeric memory as mediated by the depicted brain regions. All pcor ≥ .8. Bankssts and corpus callosum DKT regions not included in analyses.
4. Discussion
We aimed, firstly, to clarify the relationship between CRP and cognitive performance and, secondly, to assess brain morphology as potential mediators in this relationship. Our analyses of 14,512 UKBB participants, aged 40 to 70 and mostly White, found that serum CRP was associated with fluid intelligence and numeric memory, albeit with small effect sizes and no evidence for associations between serum CRP and other measures of cognitive performance. Our mediation analyses found no evidence that measures of regional brain volume, surface area, or thickness mediate these relationships. Together, our findings suggest that, for mid-to-late aged White adults, serum CRP is, at best, marginally related to certain measures of cognitive performance. Insofar as serum CRP is associated with fluid intelligence and cognitive performance, other brain metrics, including structural and functional connectivity, may be more involved in mediating these relationships. Further, given the small effect sizes of the association between serum CRP and cognitive performance, other methods of measuring CRP or other inflammation-associated-markers all together may be more relevant for cognition, a conclusion in line with findings from previous studies across various populations (Dik et al., 2005; Jordanova et al., 2007; Miralbell et al., 2012; Bora, 2019).
We found significant associations between serum CRP and both numeric memory and fluid intelligence, though the small size of these associations must be emphasized. Indeed, our results suggest that an 8.3 mg/L increase—a considerable increase that is unlikely to be maintained for long durations given CRP's acute phase response—was associated with a negligible 0.03 standard deviation change in numeric memory and fluid intelligence. The small magnitude of these significant associations may explain inconsistent reports of associations between serum CRP and these cognitive domains. Characteristics of the UKBB's cognitive tasks may also explain differences between our findings and previous works. Fawns-Ritchie & Deary (2020), in validating the UKBB cognitive tasks, found a Pearson correlation of r = 0.51 between the UKBB's numeric memory task and its reference task. Though understood to be acceptable, this association suggests that there may be considerable variability between the UKBB numeric memory task and other numeric memory tasks, perhaps explaining why we find evidence for CRP-numeric memory association despite previous reports that do not support this conclusion (Marsland et al., 2015; Beydoun et al., 2018; Luciano et al., 2009; Moreno-Navarrete et al., 2017). Regarding fluid intelligence, the UKBB's fluid intelligence score reflects the number of correct responses given in two minutes to multiple choice questions related to verbal or numeric reasoning. This test is understood to assess verbal and numeric reasoning, yet its scores correlate moderately with digit span tasks (Fawns-Ritchie and Deary, 2020). This suggests that the fluid intelligence score reflects, in part, aspects of verbal and numeric memory, perhaps explaining why this score was significantly associated with CRP in our analysis. This association supports a previous finding, albeit in a younger sample (Gimeno et al., 2008), and contrasts with analyses on a smaller sample of similarly aged participants (Luciano et al., 2009). Ultimately, the small effect sizes of serum CRP-cognitive performance associations are inconsistent with the notion of causal associations between these measures. Insofar as a causal association between CRP and cognition may exist, serum CRP and cognition seem to be distantly related at best; a conclusion in line with findings from other populations, including psychosis (Bora, 2019) and UKBB participants with mood disorders (Milton et al., 2021). Perhaps other methods of measuring CRP activity, e.g., epigenomic methods, capture CRP's effects on cognitive performance more reliably (reviewed in Walker et al., 2022) and therefore may reveal stronger relationships between CRP and cognitive performance (e.g., Conole et al., 2021). Yet, it remains unclear what mechanisms would link genetic or epigenetic markers of CRP expression to cognitive performance if serum CRP concentrations are but marginally involved.
Our analyses found associations between serum CRP and some previously associated general brain metrics but not others. Our association between serum CRP and white matter hyperintensity supports a previous analysis (Wersching et al., 2010), but, unlike other works (Marsland et al., 2015; Jefferson et al., 2007; Satizabal et al., 2012; Gu et al., 2017), we find no evidence of associations between serum CRP and whole brain volume metrics. Our findings mostly support recent work on this sample that reported no CRP-brain morphology associations (Williams et al., 2022). Differences in sample subsets, covariate selection, and corrections for multiple comparisons likely explain the differences observed between our CRP-brain morphology results and those from previous analyses on the same UKBB sample.
Given the lack of significant associations between serum CRP and brain morphology, it is unsurprising we found no support for brain morphology as mediators in the CRP-cognition relationship. While previous studies with similar mediation analyses report statistically significant mediating associations between CRP, brain morphology and cognitive performance (Bettcher et al., 2012; Marsland et al., 2015; Wersching et al., 2010), differences in the cognitive measures used likely explain these differences. Further, in our analyses, the small size of the associations between CRP and cognitive performance left little to mediate. It is plausible that grey matter volume, thickness and surface area do not capture the effects of serum CRP on the brain. Perhaps the recruitment of various brain regions in performing cognitive tasks weakens associations between cognitive performance and specific brain regions (Blazer, 2006). It may also be the case that associations between serum CRP and cognitive performance are better explained by serum CRP's effects on other aspects of the brain, e.g., vasculature (Miralbell et al., 2012), blood brain barrier integrity (Kuhlmann et al., 2009), regional brain connectivity (Shen et al., 2020), and/or neural metabolism (Gregor and Hotamisligil, 2011).
4.1. Strengths and limitations
The 14,512 participants with complete data represent one of the largest samples on which associations between CRP, cognition, and brain morphology have been assessed. Such a sample size provided adequate power to detect even the smallest of effects. Further, the UKBB provided comprehensive assessments of serum CRP levels, cognitive performance, brain morphology, and potential confounding factors. Confounding factors from multiple time points were included in our analyses providing robust control for these factors. Further, indices of model fit suggest that mediation models are well fit to the data, providing evidence against multicollinearity in these analyses. The public availability of our code and the availability of UKBB to researchers facilitates the reproducibility of our results.
These analyses used serum CRP levels from a single time point. Considering the multi-year delay between collection of the blood sample at baseline and the imaging and cognitive assessments performed at follow-up 2, it is unclear how well this single measure of serum CRP represents individual's basal levels; this limitation may help explain the lack of associations found in our primary analyses. While our preliminary analyses showed stronger longitudinal associations between serum CRP concentrations taken multiple years apart in the UKBB data than reported in a recent meta-analysis (Walsh et al., 2023), aggregating serum CRP measures from multiple time points could be of great value. Unfortunately, using multiple serum CRP measures was not possible in these analyses as CRP data at follow-up 1 were limited in number and follow-up 2 CRP data were unavailable. Moreover, CRP concentrations were measured from peripheral blood samples. Given this study's interest in the possible effect of CRP on the central nervous system and considering the complex associations between fluids in peripheral tissues and those in the central nervous system, future studies should consider the trade-off between more invasive measures of CRP levels in central nervous system fluids and increased precision for brain-immunity studies. Finally, the UKBB performed aliquots on CRP levels but on few other inflammation-related serum cytokines. It would be informative to perform these analyses on additional cytokines, including IL-6 – a cytokine that promotes CRP production (Pearson et al., 2003).
The generalizability of these results may be limited given participant characteristics of the UKBB. First, the UKBB's sample is aged between 40 and 70 at baseline, as such these results may not apply beyond this age group. As has been previously established, UKBB participants differ from the general UK population on several variables. Among these differences, fewer UKBB participants smoke and fewer use SSRIs than the general UK population (Fry et al., 2017; Taylor et al., 2019). Moreover, the results reported in this manuscript are for a specific subset of the participants, namely those not using SSRIs and not diagnosed with specified medical diagnoses. Nonetheless, results from our supplementary analyses on different subsets of this sample are not qualitatively different than those reported here—certainly not in terms of effect sizes of the significant correlations—and are consistent with findings on other populations, as noted above.
5. Conclusions
Our comprehensive analyses of over 14,000 well-characterised participants from the UKBB, that rigorously control for numerous potentially confounding factors, finds that serum CRP appears to have a marginal association with measures of fluid intelligence and numeric memory. Brain morphology does not appear to mediate these relationships. Future studies on CRP would be well to assess connectivity-based brain measures or functional metrics as potential mediators as well as to consider other methods of measuring CRP activation, like using epigenetic methods. Other inflammation-associated markers, like IL-6, may also be more pertinent for cognition and brain structure.
Funding
This work did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Mr. Joshua Unrau provided valuable support for this project.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2023.100664.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The authors do not have permission to share data.
References
- Alfaro-Almagro F., Jenkinson M., Bangerter N.K., et al. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. Neuroimage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M.E., Teixeira A.L. Inflammation in psychiatric disorders: what comes first? Ann. N. Y. Acad. Sci. 2019;1437(1):57–67. doi: 10.1111/nyas.13712. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. Roy. Stat. Soc. 1995;57(1):289–300. 1995. [Google Scholar]
- Bettcher B.M., Wilheim R., Rigby T., et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav. Immun. 2012;26(1):103–108. doi: 10.1016/j.bbi.2011.07.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beydoun M.A., Dore G.A., Canas J.A., Liang H., Beydoun H.A., Evans M.K., Zonderman A.B. Systemic inflammation is associated with longitudinal changes in cognitive performance among urban adults. Front. Aging Neurosci. 2018;10:313. doi: 10.3389/fnagi.2018.00313. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer D.G. Cognitive neuroscience of aging: linking cognitive and cerebral aging. Aust. J. Pharm. 2006;163(3):560–561. 2006. [Google Scholar]
- Bora E. Peripheral inflammatory and neurotrophic biomarkers of cognitive impairment in schizophrenia: a meta-analysis. Psychol. Med. 2019;49(12):1971–1979. doi: 10.1017/S0033291719001685. [DOI] [PubMed] [Google Scholar]
- Brem A.K., Ran K., Pascual-Leone A. Learning and memory. Handb. Clin. Neurol. 2013;116:693–737. doi: 10.1016/B978-0-444-53497-2.00055-3. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conole E.L., Stevenson A.J., Maniega S.M., et al. DNA methylation and protein markers of chronic inflammation and their associations with brain and cognitive aging. Neurology. 2021;97(23):e2340–e2352. doi: 10.1212/WNL.0000000000012997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S.R., Ritchie S.J., Fawns-Ritchie C., Tucker-Drob E.M., Deary I.J. Structural brain imaging correlates of general intelligence in UK Biobank. Intelligence. 2019;76 doi: 10.1016/j.intell.2019.101376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale A.M., Fischl B., Sereno M.I. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9(2):179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dik M.G., Jonker C., Hack C.E., Smit J.H., Comijs H.C., Eikelenboom P. Serum inflammatory proteins and cognitive decline in older persons. Neurology. 2005;64(8):1371–1377. doi: 10.1212/01.WNL.0000158281.08946.68. [DOI] [PubMed] [Google Scholar]
- Durlak J.A. How to select, calculate, and interpret effect sizes. J. Pediatr. Psychol. 2009;34(9):917–928. doi: 10.1093/jpepsy/jsp004. [DOI] [PubMed] [Google Scholar]
- Fawns-Ritchie C., Deary I.J. Reliability and validity of the UK Biobank cognitive tests. PLoS One. 2020;15(4) doi: 10.1371/journal.pone.0231627. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B. FreeSurfer. NeuroImage. 2012;62(2):774–781. doi: 10.1016/j.neuroimage.2012.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourrier C., Singhal G., Baune B.T. Neuroinflammation and cognition across psychiatric conditions. CNS Spectr. 2019;24(1):4–15. doi: 10.1017/S1092852918001499. [DOI] [PubMed] [Google Scholar]
- Frodl T., Amico F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2014;48:295–303. doi: 10.1016/j.pnpbp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- Fry A., Littlejohns T.J., Sudlow C., Doherty N., Adamska L., Sprosen T., Collins R., Allen N.E. Comparison of sociodemographic and health-related characteristics of UK biobank participants with those of the general population. Am. J. Epidemiol. 2017;186(9):1026–1034. doi: 10.1093/aje/kwx246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry D., Almond R., Moffat S., Gordon M., Singh P. UK Biobank; 2019. UK Biobank Biomarker Project: Companion Document to Accompany Serum Biomarker Data. [Google Scholar]
- Gabay C., Kushner I. Acute-phase proteins and other systemic responses to inflammation. N. Engl. J. Med. 1999;340(6):448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- Gimeno D., Marmot M.G., Singh-Manoux A. Inflammatory markers and cognitive function in middle-aged adults: the Whitehall II study. Psychoneuroendocrinology. 2008;33(10):1322–1334. doi: 10.1016/j.psyneuen.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor M.F., Hotamisligil G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011;29(1):415–445. doi: 10.1146/annurev-immunol-031210-101322. 2011. [DOI] [PubMed] [Google Scholar]
- Gu Y., Vorburger R., Scarmeas N., Luchsinger J.A., Manly J.J., Schupf N., Mayeux R., Brickman A.M. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun. 2017;65:150–160. doi: 10.1016/j.bbi.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagstromer M., Oja P., Sjostrom M. The International Physical Activity Questionnaire (IPAQ): a study of concurrent and construct validity. Publ. Health Nutr. 2006;9(6):755–762. doi: 10.1079/phn2005898. [DOI] [PubMed] [Google Scholar]
- Jefferson A.L., Massaro J.M., Wolf P.A., et al. Inflammatory biomarkers are associated with total brain volume: the Framingham Heart Study. Neurology. 2007;68(13):1032–1038. doi: 10.1212/01.wnl.0000257815.20548.df. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordanova V., Stewart R., Davies E., Sherwood R., Prince M. Markers of inflammation and cognitive decline in an African-Caribbean population. Int. J. Geriatr. Psychiatr. 2007;22(10):966–973. doi: 10.1002/gps.1772. [DOI] [PubMed] [Google Scholar]
- Kaup A.R., Mirzakhanian H., Jeste D.V., Eyler L.T. A review of the brain structure correlates of successful cognitive aging. J Neuropsychiatry Clin Neurosci Winter. 2011;23(1):6–15. doi: 10.1176/appi.neuropsych.23.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil M., Hollander P., Raucher-Chene D., Lepage M., Lavigne K.M. Structural brain correlates of cognitive function in schizophrenia: a meta-analysis. Neurosci. Biobehav. Rev. 2022;132:37–49. doi: 10.1016/j.neubiorev.2021.11.034. [DOI] [PubMed] [Google Scholar]
- Klein A., Tourville J. 101 labeled brain images and a consistent human cortical labeling protocol. Front. Neurosci. 2012;6:171. doi: 10.3389/fnins.2012.00171. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlmann C.R., Librizzi L., Closhen D., Pflanzner T., Lessmann V., Pietrzik C.U., de Curtis M., Luhmann H.J. Mechanisms of C-reactive protein-induced blood-brain barrier disruption. Stroke. 2009;40(4):1458–1466. doi: 10.1161/STROKEAHA.108.535930. [DOI] [PubMed] [Google Scholar]
- Lai K.S.P., Liu C.S., Rau A., Lanctot K.L., Kohler C.A., Pakosh M., Carvalho A.F., Herrmann N. Peripheral inflammatory markers in Alzheimer's disease: a systematic review and meta-analysis of 175 studies. J. Neurol. Neurosurg. Psychiatry. 2017;88(10):876–882. doi: 10.1136/jnnp-2017-316201. [DOI] [PubMed] [Google Scholar]
- Luciano M., Marioni R.E., Gow A.J., Starr J.M., Deary I.J. Reverse causation in the association between C-reactive protein and fibrinogen levels and cognitive abilities in an aging sample. Psychosom. Med. 2009;71(4):404–409. doi: 10.1097/PSY.0b013e3181a24fb9. [DOI] [PubMed] [Google Scholar]
- Marsland A.L., Gianaros P.J., Kuan D.C., Sheu L.K., Krajina K., Manuck S.B. Brain morphology links systemic inflammation to cognitive function in midlife adults. Brain Behav. Immun. 2015;48:195–204. doi: 10.1016/j.bbi.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan M.J., Agid Y., Brune M., et al. Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat. Rev. Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- Milton D.C., Ward J., Ward E., Lyall D.M., Strawbridge R.J., Smith D.J., Cullen B. The association between C-reactive protein, mood disorder, and cognitive function in UK Biobank. Eur. Psychiatr. 2021;64(1):e14. doi: 10.1192/j.eurpsy.2021.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miralbell J., Soriano J.J., Spulber G., et al. Structural brain changes and cognition in relation to markers of vascular dysfunction. Neurobiol. Aging. 2012;33(5):1003 e1009–e1017. doi: 10.1016/j.neurobiolaging.2011.09.020. [DOI] [PubMed] [Google Scholar]
- Moreno-Navarrete J.M., Blasco G., Puig J., et al. Neuroinflammation in obesity: circulating lipopolysaccharide-binding protein associates with brain structure and cognitive performance. Int. J. Obes. 2017;41(11):1627–1635. doi: 10.1038/ijo.2017.162. [DOI] [PubMed] [Google Scholar]
- Noble J.M., Manly J.J., Schupf N., Tang M.X., Mayeux R., Luchsinger J.A. Association of C-reactive protein with cognitive impairment. Arch. Neurol. 2010;67(1):87–92. doi: 10.1001/archneurol.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor M.F., Bower J.E., Cho H.J., et al. To assess, to control, to exclude: effects of biobehavioral factors on circulating inflammatory markers. Brain Behav. Immun. 2009;23(7):887–897. doi: 10.1016/j.bbi.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson T.A., Mensah G.A., Alexander R.W., et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107(3):499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pepys M.B., Hirschfield G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafnsson S.B., Deary I.J., Smith F.B., Whiteman M.C., Rumley A., Lowe G.D., Fowkes F.G. Cognitive decline and markers of inflammation and hemostasis: the Edinburgh Artery Study. J. Am. Geriatr. Soc. 2007;55(5):700–707. doi: 10.1111/j.1532-5415.2007.01158.x. [DOI] [PubMed] [Google Scholar]
- Satizabal C.L., Zhu Y.C., Mazoyer B., Dufouil C., Tzourio C. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 2012;78(10):720–727. doi: 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- Schram M.T., Euser S.M., de Craen A.J., et al. Systemic markers of inflammation and cognitive decline in old age. J. Am. Geriatr. Soc. 2007;55(5):708–716. doi: 10.1111/j.1532-5415.2007.01159.x. [DOI] [PubMed] [Google Scholar]
- Shen J., Tozer D.J., Markus H.S., Tay J. Network efficiency mediates the relationship between vascular burden and cognitive impairment: a diffusion tensor imaging study in UK biobank. Stroke. 2020;51(6):1682–1689. doi: 10.1161/STROKEAHA.119.028587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Alfaro-Almagro F., Miller K.L. UK Biobank; 2020. UK Biobank Brain Imaging Documentation. [Google Scholar]
- Sommer I., Ramsey N., Kahn R., Aleman A., Bouma A. Handedness, language lateralisation and anatomical asymmetry in schizophrenia: meta-analysis. Br. J. Psychiatry. 2001;178(4):344–351. doi: 10.1192/bjp.178.4.344. [DOI] [PubMed] [Google Scholar]
- Taki Y., Thyreau B., Kinomura S., et al. Correlation between high-sensitivity C-reactive protein and brain gray matter volume in healthy elderly subjects. Hum. Brain Mapp. 2013;34(10):2418–2424. doi: 10.1002/hbm.22073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tampubolon G. Repeated systemic inflammation was associated with cognitive deficits in older Britons. Alzheimers Dement (Amst) 2016;3:1–6. doi: 10.1016/j.dadm.2015.11.009. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor S., Annand F., Burkinshaw P., et al. Public Health England; 2019. Dependence and Withdrawal Associated with Someprescribed Medicines: an Evidence Review. [Google Scholar]
- Tegeler C., O'Sullivan J.L., Bucholtz N., Goldeck D., Pawelec G., Steinhagen-Thiessen E., Demuth I. The inflammatory markers CRP, IL-6, and IL-10 are associated with cognitive function--data from the Berlin Aging Study II. Neurobiol. Aging. 2016;38:112–117. doi: 10.1016/j.neurobiolaging.2015.10.039. [DOI] [PubMed] [Google Scholar]
- Townsend P. Deprivation. J. Soc. Pol. 1987;16(2):125–146. [Google Scholar]
- van den Berg E., Kloppenborg R.P., Kessels R.P., Kappelle L.J., Biessels G.J. Type 2 diabetes mellitus, hypertension, dyslipidemia and obesity: a systematic comparison of their impact on cognition. Biochim. Biophys. Acta. 2009;1792(5):470–481. doi: 10.1016/j.bbadis.2008.09.004. [DOI] [PubMed] [Google Scholar]
- Walker K.A., Basisty N., Wilson D.M., 3rd, Ferrucci L. Connecting aging biology and inflammation in the omics era. J. Clin. Invest. 2022;132(14) doi: 10.1172/JCI158448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh C.P., Lindsay E.K., Grosse P., et al. A systematic review and meta-analysis of the stability of peripheral immune markers in healthy adults. Brain Behav. Immun. 2023;107:32–46. doi: 10.1016/j.bbi.2022.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren K.N., Beason-Held L.L., Carlson O., et al. Elevated markers of inflammation are associated with longitudinal changes in brain function in older adults. J Gerontol A Biol Sci Med Sci. 2018;73(6):770–778. doi: 10.1093/gerona/glx199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wersching H., Duning T., Lohmann H., et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- Weuve J., Ridker P.M., Cook N.R., Buring J.E., Grodstein F. High-sensitivity C-reactive protein and cognitive function in older women. Epidemiology. 2006;17(2):183–189. doi: 10.1097/01.ede.0000198183.60572.c9. [DOI] [PubMed] [Google Scholar]
- Williams J.A., Burgess S., Suckling J., et al. Inflammation and brain structure in schizophrenia and other neuropsychiatric disorders: a mendelian randomization study. JAMA Psychiatr. 2022;79(5):498–507. doi: 10.1001/jamapsychiatry.2022.0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K., Lindquist K., Penninx B.W., et al. Inflammatory markers and cognition in well-functioning African-American and white elders. Neurology. 2003;61(1):76–80. doi: 10.1212/01.wnl.0000073620.42047.d7. [DOI] [PubMed] [Google Scholar]
- Zimmerman M.E., Brickman A.M., Paul R.H., et al. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. Am. J. Geriatr. Psychiatr. 2006;14(10):823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors do not have permission to share data.