Figure 1.
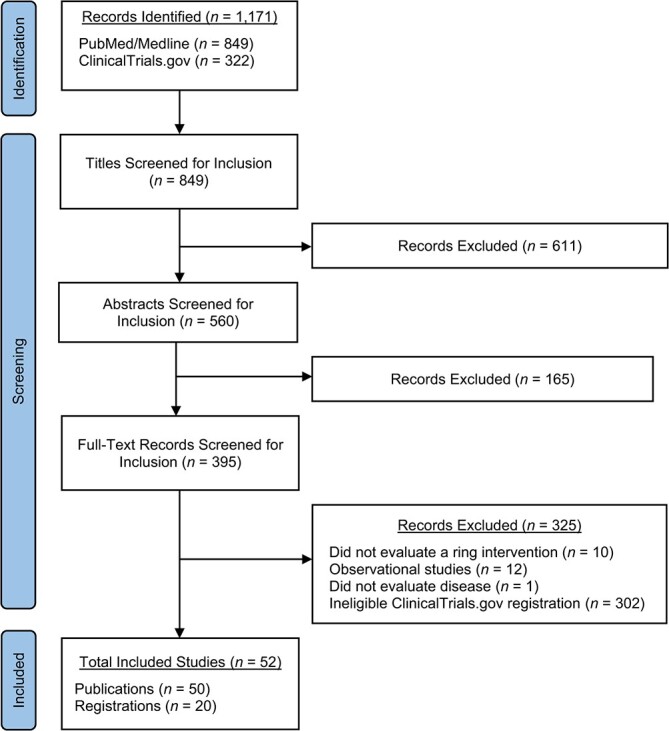
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram for systematic review screening and inclusion. All registrations from ClinicalTrials.gov were reviewed in a single stage of full-text review, and records overlapped. “Total included studies” refers to research projects for which 1 or more records were included. Records of studies include trial registrations, published trial protocols, and original research articles.
