Figure 6. ALK fusions are sensitive to ALK inhibitors.
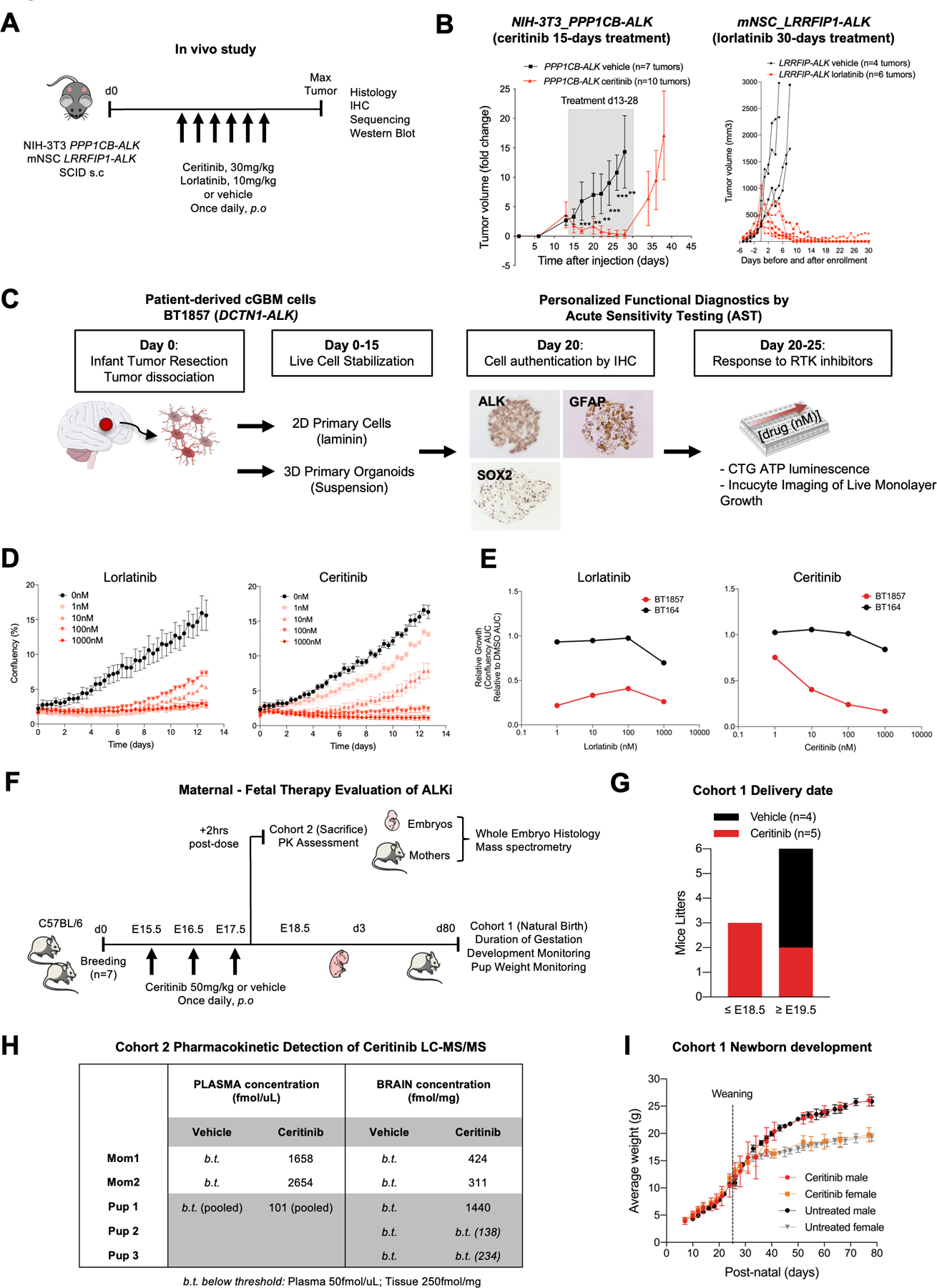
A. Study schematic for subcutaneous transplantation of PPP1CB-ALK-NIH-3T3 and LRRFIP-ALK-mNSC in SCID mice. Ceritinib and Lorlatinib treatments were initiated when tumor volume reached 200 +/− 200mm3 and continued for 15 and 30 consecutive days respectively.
B. Caliper measurements of tumor volume in mice treated with vehicle (black curves) or ALK inhibitors (red curves). Shaded area denotes treatment range. Data are fold change +/− s.e.m of controls.
C. Personalized functional diagnostic approach and acute sensitivity testing of cGBM cells (BT1857), bearing a DCTN1-ALK fusion. Live cells were isolated from the newborn tumor, dissociated, and stabilized for up to 15 days in culture as organoid/spheroid or 2D adherent cultures on laminin prior to authentication by IHC. Cells were tested for sensitivity to ALK and other kinase inhibitors by CellTiterGlo or Incucyte monospheroid/organoid growth assays.
D. Incucyte growth profile of adherent cGBM DCTN1-ALK fused cells (BT1857) showing dose response to ALK inhibitors lorlatinib and ceritinib. Incucyte raw growth curves show confluency (%) over ~13 days of continuous imaging.
E. DCTN1-ALK fused patient cells (BT1857) sensitivity to ALKi compared to an ALK wild-type GBM cell line (BT164). AUC curves of ALKi-treated cells versus DMSO control.
F. Schematic outlining the administration of a brain penetrant ALK inhibitor, Ceritinib, to pregnant C57BL/6 mice to assess pharmacokinetics and neural developmental effects in perinatal period.
G. Delivery dates of live pups for pregnant C57BL/6 mice treated with vehicle or ceritinib from E15.5 to E17.5 embryonic development stages.
H. Quantification of ceritinib concentrations by LC-MS/MS in maternal, neonatal brain, plasma, and liver.
I. Newborn’s weight in control and ceritinib-treated groups.
