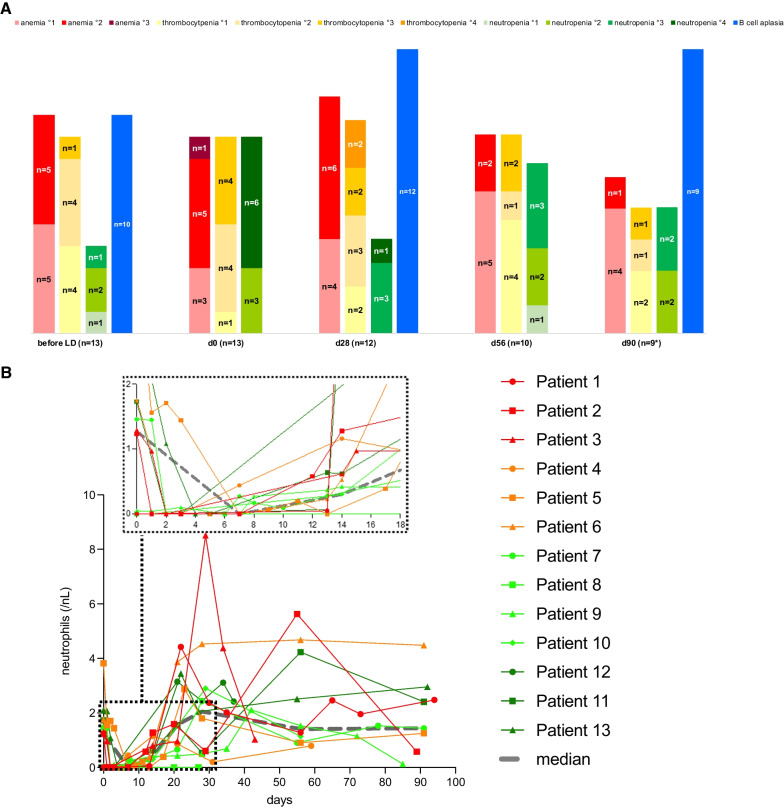
Fig. 2.
Hematologic toxicity of HD-CAR-1 treatment. A Cytopenia and B cell aplasia. On day 0, i.e., after lymphodepletion (LD) and before CART administration, 69% (n = 9) of patients were neutropenic (46% grade IV neutropenia), anemic (8% grade III anemia) and thrombocytopenic (31% grade III thrombocytopenia). One month after HD-CAR-1 treatment, 8% (n = 1, UPN#4) patients displayed grade IV neutropenia and 17% (n = 2; UPN#4 and UPN#11) grade IV thrombocytopenia. On end-of-study (EOS) at day 90, two patients showed persistent grade III neutropenia (UPN#2, UPN #4) and one patient grade III thrombocytopenia (UPN#4) despite treatment with granulocyte-colony-stimulating factor (G-CSF) and a thrombopoietin-agonist, respectively. (UPN#4 had grade III neutropenia and thrombocytopenia already before receiving CARTs; also, UPN#2 was already neutropenic before CART treatment.) No higher-grade anemia was observed. Beyond day 28, no grade IV cytopenia was observed. As for B cell counts, 77% (n = 10) of patients displayed B cell aplasia already before receiving CARTs. At EOS, all evaluable patients (n = 9; UPN#9 not shown due to PD) had ongoing B cell aplasia (B cell count on day 0 and day 56 not assessed). B Absolute neutrophil count (ANC) of treated HD-CAR-1 patients (n = 13) within the first 18 days (top, small frame) and up to end of study on day 90 after CART treatment. Four patients (UPN #1, #3, #4, #11, #13) received G-CSF after CARTs. Median of ANCs is depicted in grey