Abstract
The most significant challenge for nucleic acid drug development is their delivery across the cell membrane. Herein, we harness the reversible binding between boronic acids and cell surface glycans to aid in the cellular delivery of synthetic oligonucleotides. We install the artificial nucleotide 5-dihydroxyboryluridine (5boU) in a site-specific manner within druglike antisense oligonucleotides and demonstrate that these boronate-containing nucleic acids have enhanced cytosolic penetration and splice-correcting activity compared to non-boronate analogs. Strategic incorporation of 5boU is a simple, modular, and potentially general means of enhancing cellular delivery of therapeutic nucleic acids.
Graphical Abstract
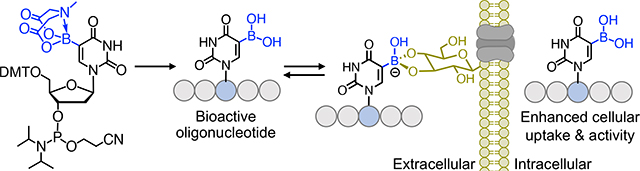
Boronic acid functionalized synthetic antisense oligonucleotides demonstrate enhanced cellular uptake and biological activity, constituting a novel approach for delivery of therapeutic oligonucleotides
Nucleic acid therapeutics, including antisense oligonucleotides (ASOs), short interfering RNAs (siRNAs), microRNAs (miRNAs), and mRNAs is a promising and growing area in drug development.1, 2 Short, synthetic nucleic acid therapeutics such as ASOs are chemically modified, for example with 2’-O-methylated (2’-OCH3) sugars and a phosphorothioate (PS) backbone, to confer nuclease resistance and improve pharmacokinetics.3 However, the limited cell permeability of ASOs considerably hinders their use as chemical probes and therapeutic agents. Thus, the single greatest obstacle for nucleic acid therapeutics is their delivery across the plasma membrane.4
Several strategies have been developed to enhance cellular delivery of ASOs and related oligonucleotides. The most common methods use polycationic cell-penetrating peptides (CPPs) that are covalently conjugated to oligonucleotide cargos via thioether, thiol-maleimide, or click ligation.5 Derivatization with a cleavable linkage, such as a disulfide bond, has also been employed for peptide-mediated delivery both in cells and in vivo. Another delivery strategy exploits the noncovalent interactions between cationic CPPs and negatively charged ASOs and siRNAs.6 Furthermore, peptide vectors containing hydrophobic elements (fatty acids, lipids, or cholesterol) can form complexes with oligonucleotides which translocate them across the plasma membrane.7 However, for all these strategies, issues related to chemical modifications, aggregation via charge neutralization, cell-type dependent heterogeneous uptake, and ineffective endosomal escape are common.
Electrophilic boronic acids form reversible, cyclic boronate esters with 1,2- and 1,3-diols, including those presented by glycans at the cell surface.10–13 Prior work has shown that the dynamic covalent bonding between boronic acid and cell-surface glycans can promote cellular internalization and endosomal escape for the delivery of bioactive peptides and proteins.8, 9 We hypothesized that installing boronic acids within ASOs could offer an effective strategy for promoting their cytosolic delivery (Fig. 1A). We recently developed a concise synthetic strategy to prepare and incorporate phosphoramidite of 5-dexyboryldexoyuridine (5boU) into short oligonucleotides using solid-phase chemistry.14 In this work, we install 5boU in a panel of druglike oligonucleotides and examine their cytosolic penetration and cellular activity.
Fig. 1.
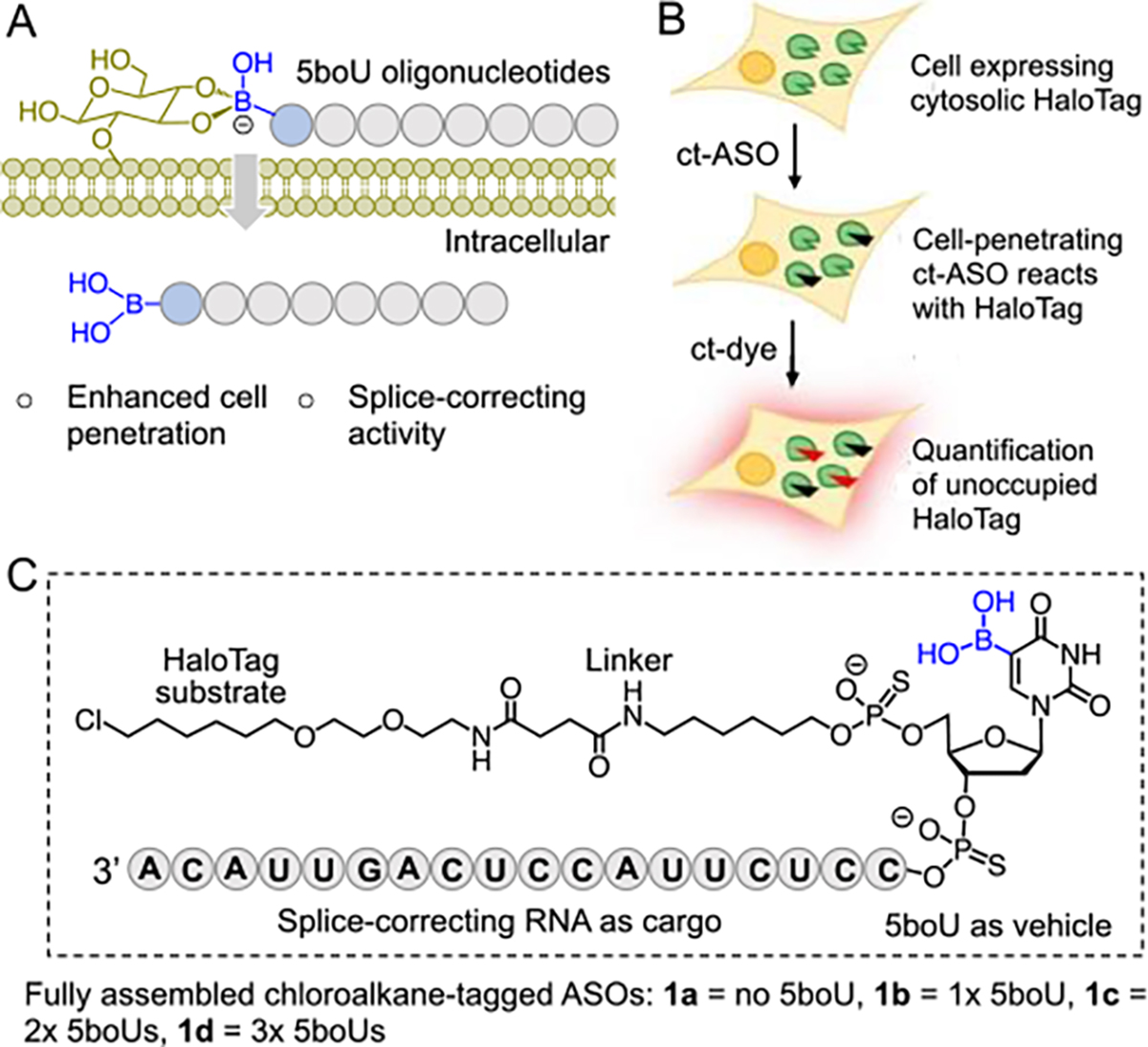
Model for enhanced cell penetration of boronic acid containing ASOs. (A) Enhanced internalization of 5boU-containing nucleic acids is envisioned to occur via reversible covalent linking with cell-surface glycans, as previously described for boronate-containing peptides and proteins.8, 9 (B) Schematic showing chloroalkane penetration assay (CAPA). (C) Structure of test compounds consisting of a druglike ASO, variable 5boU moieties, a linker, and a HaloTag substrate. ct-ASO: chloroalkane-tagged antisense oligonucleotide.
To quantitatively measure cytosolic penetration of the chemically modified nucleic acids, we employed the chloroalkane penetration assay (CAPA) (Fig. 1B).15, 16 In CAPA, cells expressing HaloTag enzyme are treated with a chloroalkane-tagged (ct) compound, in this case a ct-ASO, then chased with ct-dye. ct-ASO that crosses the cell membrane will covalently block HaloTag, preventing the dye from being retained by cells in the chase step. Thus, the degree to which the ct-ASO penetrates the cytosol is inversely proportional to the fluorescence signal obtained after the chase step. Dose-dependent CAPA provides a sigmoidal curve which can be fit to produce a CP50 value, the concentration of ct-ASO at which 50% of HaloTag is blocked. CP50 values have proven to be highly quantitative and reproducible measurements of cytosolic delivery.16 This method has been applied to a variety of large-molecule therapeutics, including ASOs,17 and allows for direct measurement of cell penetration without interference from endosomally trapped material.
To apply CAPA to measure the effects of 5boU on membrane permeability, we designed a panel of compounds (1a-d) consisting of (i) a chloroalkyl substrate for HaloTag, (ii) a linker, (iii) a 5boU-containing unit, and (iv) an ASO cargo (Fig. 1C). The ASO was prepared with 2’-O-methylated (2’-OCH3) sugars and phosphorothioate (PS) backbone to provide stability against nucleases– a combination of chemical modifications that matches those of FDA-approved ASO drugs as well as those in clinical trials.18 The sequence of the ASOs was chosen to promote splice correction of a luciferase reporter system.19, 20
We developed a convergent synthetic method to assemble the modified ASOs (Scheme 1, 2). The chloroalkyl HaloTag substrate moiety was prepared as an activated N-hydroxysuccinimide (NHS) ester 2 from commercially available 3 following a four-step protocol (Scheme 1A, Fig. S1–5).21, 22 Briefly, 3 was subjected to chemoselective alkylation with 1-chloro-6-iodohexane to afford iodo derivative 4. Subsequently, acid-catalyzed deprotection of Boc-carbamate led to free amine 5 which was coupled with succinic acid to furnish 6, and finally, NHS esterification of 6 led to 2. To introduce the long-chain alkyl linker, we employed trifluoroacetyl protected hexylamine phosphoramidite 7 (Scheme 1B).
Scheme 1.
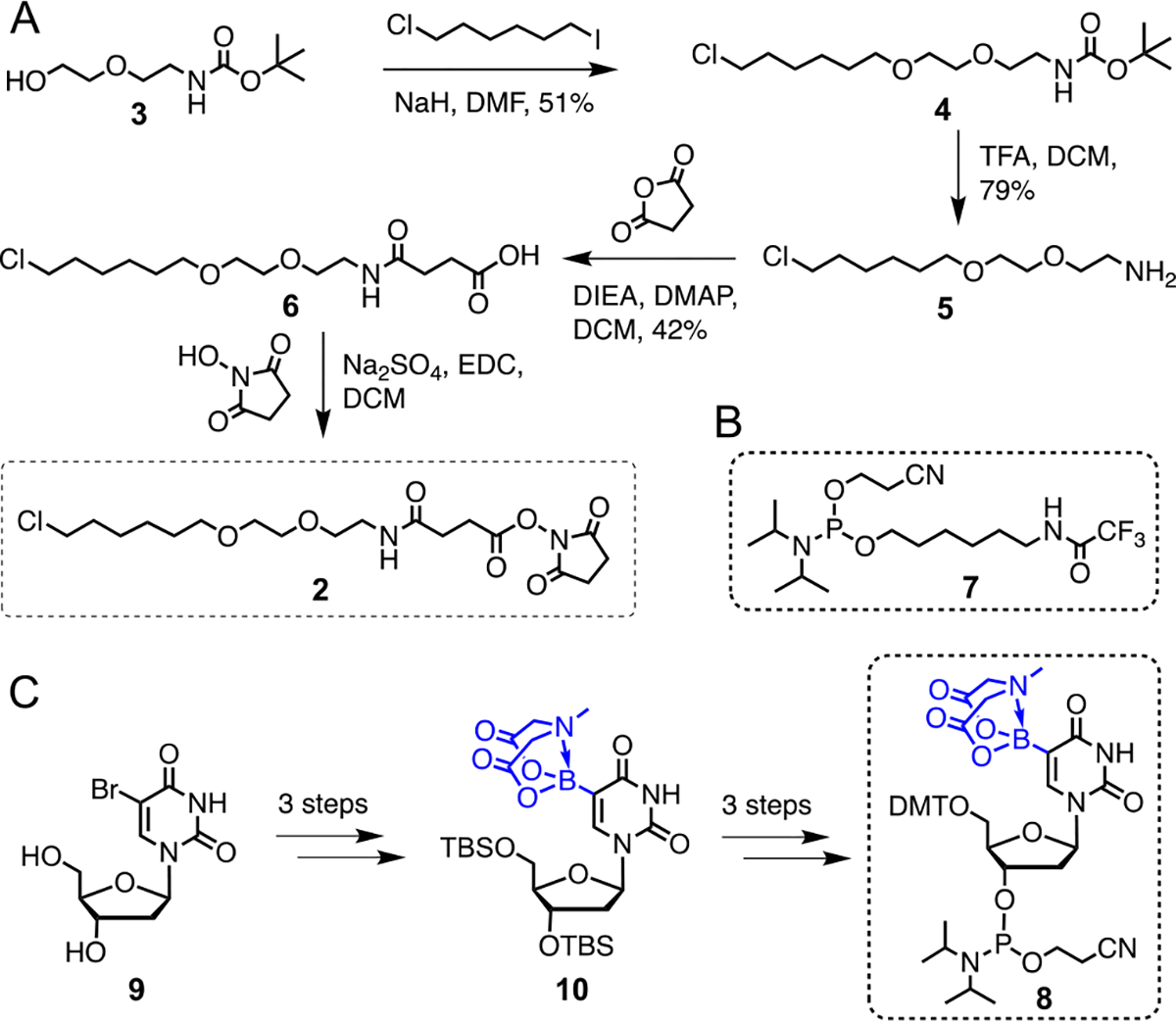
Synthesis of the individual components 2, 7 and 8. (A) Synthetic scheme for 2. (B) Structure of linker phosphoramidite 7. (C) Synthetic scheme for 8 based on the previously reported approach.14
Scheme 2.
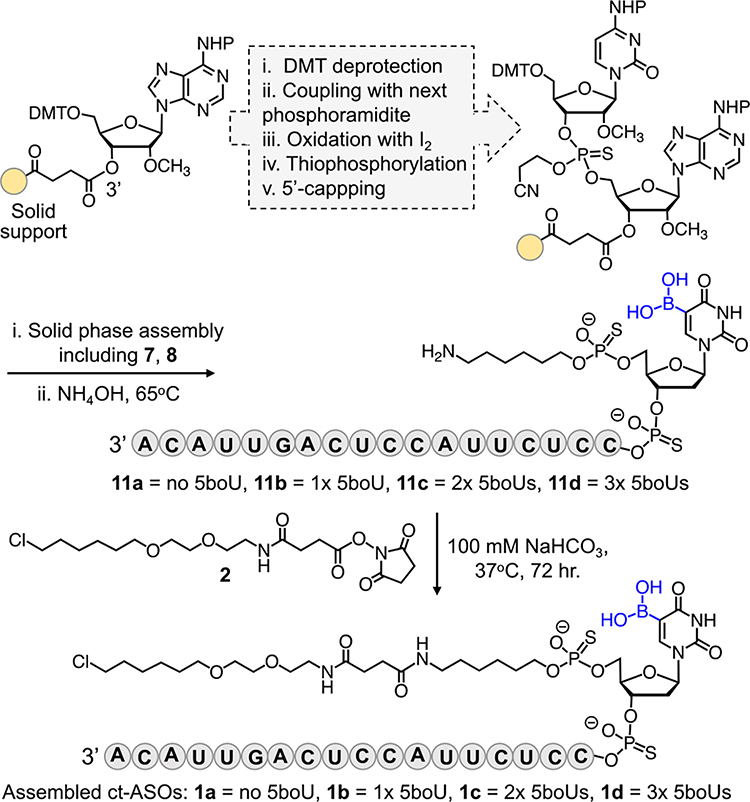
Synthesis of the fully assembled ASOs. Shown are the chemical steps to synthesize a dinucleotide with thiophosphate linkage via solid-phase technique. The cycle is repeated to incorporate the remaining canonical bases as well as 7 and 8. Ammonia-mediated global deprotection and cleavage led to unprotected 11a-d which were subsequently reacted with 2 to furnish the final compounds 1a-d.
To construct the boronic acid component, we synthesized N-methyliminodiacetic acid (MIDA)-protected 5boU phosphoramidite building block 8 following our previously reported strategy (Scheme 1C).14 Briefly, 5-bromouridine 9 was treated with TBS chloride followed by metal-halogen exchange mediated installation of the boronic acid moiety as the key step, and MIDA protection furnished an advanced intermediate 10. Subsequent protection-deprotection steps and phosphoramidation led to phosphoramidite 8 (Scheme 1C). The rationale to employ a base-sensitive MIDA protecting group is that it remains intact during trichloroacetic acid mediated cleavage of 5-dimethoxytrityl (DMT) groups during solid-phase DNA synthesis. Furthermore, MIDA can be removed by ammonia while performing global deprotection and release of the completed oligonucleotide from the solid support. The current work represents the first demonstration of site-specific incorporation of 5boU within bioactive nucleic acids.
Upon accessing the 5boU phosphoramidite 8, we proceeded to synthesize 2‘-OCH3-modified, PS-modified ASOs 11a-d with a free amine at the 5’ end (Scheme 2). 5boU units were installed at the 5’ end of the ASOs to allow for late-stage incorporation of the boronate-containing nucleotides, and because substitution at the 5’ end is expected to minimally perturb the splice-correcting activity of the ASOs. To introduce phosphorothioate linkages, the growing oligonucleotide sequence was treated with 3-[(Dimethylaminomethylene)amino]-3H-1,2,4-dithiazole-5-thione (DDTT)23 in each cycle during solid-phase synthesis, demonstrating compatibility of boronic acid with DDTT which is frequently used in the synthesis of phosphorothioated ribonucleic acids. Employing this optimized reaction sequence, we synthesized a panel of ASOs carrying up to three 5boU units to systematically examine the effects of boronic acid on cytosolic penetration.
After incorporation of 5boU, we attached the long-chain alkyl linker to the oligonucleotides on a solid support using phosphoramidite 7 (Scheme 2). Ammonia-mediated global deprotection and cleavage from the solid support followed by HPLC purification yielded analytically pure compounds 11a-d (Fig. S6–13). Subsequently, the free primary amine was conjugated with the chloroalkane-NHS ester 2 in solution to yield the final modified ASOs 1a-d which were further purified by HPLC (Scheme 2). The identity of each intermediate and final compound was confirmed by ESI-HRMS (Fig. 2, S6–13).
Fig. 2.
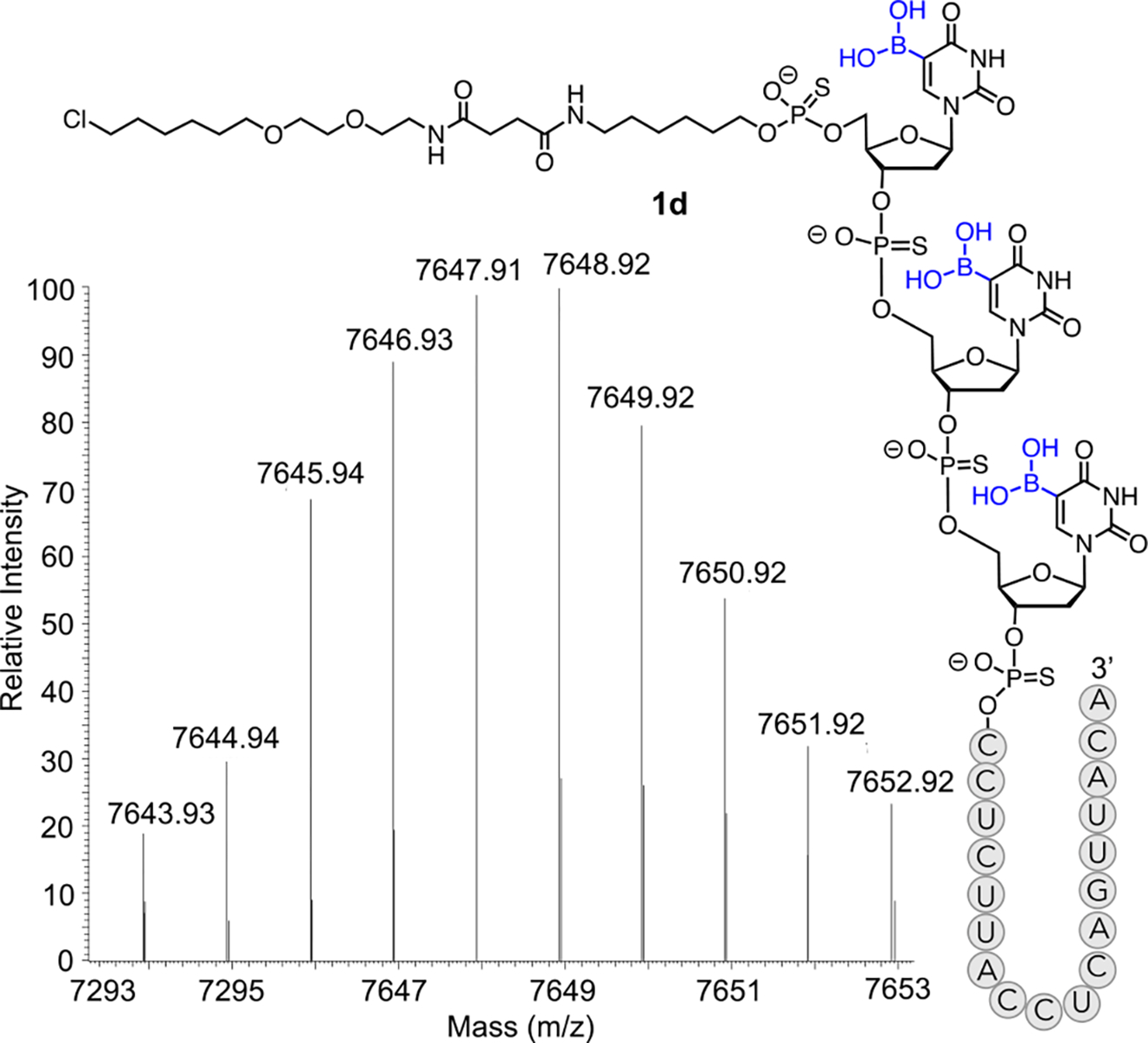
Deconvoluted LC-HRMS spectrum of fully assembled ASO 1d carrying three 5boU units. Mass spectrometry data for additional ASOs are provided in Supplementary Figures S6–13.
After successfully synthesizing the fully assembled ct-ASOs, we proceeded to obtain CAPA data for each of the compounds at concentrations ranging from 0.5 nM to 1 μM (Fig. 3A). The CP50 value for control small molecule chloroalkane tagged-tryptophan (ct-W) was measured at 0.043 μM, consistent with prior observation (Fig. 3A, S14).17 The CP50 value for the non-boronate-containing ct-ASO 1a was 0.43 ± 0.11 μM, consistent with results previously observed for similar PS,2ʹ-O-methyoxyethyl-modified ASOs.17 The CP50 value for an identical ct-ASO with a single 5boU 1b was 0.18 ± 0.06 μM, which means a single boronate moiety conferred a 2.4-fold increase in cytosolic penetration. The CP50 values for ct-ASOs with two and three 5boU nucleotides, 1c and 1d, respectively, were 0.074 ± 0.004 μM and 0.057 ± 0.015 μM, respectively, indicating a further increase in cell permeability of roughly 2.4-fold for the second boronate moiety, followed by diminishing improvement when a third boronate was added (Fig. 3A). Overall, adding two or three 5boU residues improved cell permeability by over 5-fold, resulting in cytosolic penetration that was nearly as efficient as a small molecule.
Fig. 3.
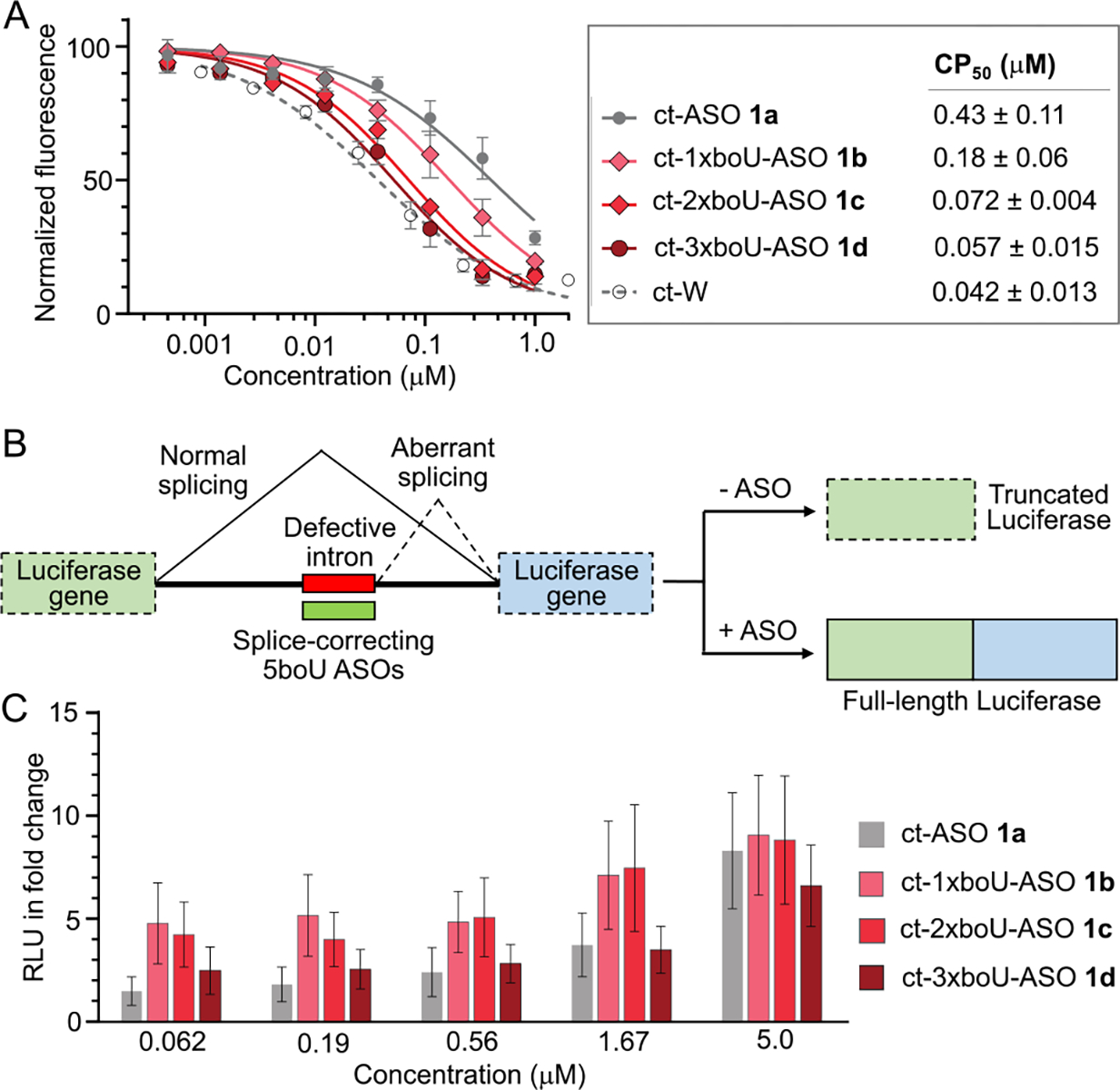
Cytosolic penetration and biological activity of boronate-modified ASOs and controls. (A) Data from chloroalkane penetration assay using HaloTag-expressing HeLa cells.16 ct-ASOs and control small molecule ct-W were incubated with cells at indicated concentrations for 4 h, followed by washes and a chase with ct-dye. CP50 values were calculated based on the curve fits shown.17 Error bars and CP50 errors are standard errors of the mean from at least three independent trials. (B) Schematic showing splice-correction based luciferase reporter assay.19 (C) Data from splice-correcting reporter assay using HeLa cells.17 All data are reported in terms of fold change of relative luminescence units (RLU) compared to untreated cells. Error bars show standard errors of the mean from three independent trials.
To correlate the CAPA data to ASO function, we then tested the ASOs in an established cell-based luciferase reporter assay (Fig. 3B).17, 19 In this assay, ASO corrects exon splicing of a β-globin intron inserted into a luciferase-coding mRNA, such that ASO splice-correction leads to expression of full-length luciferase. Relative luminescence units (RLU) of ct-ASO-treated cells were normalized to the background as measured for untreated cells. ct-ASOs were tested at concentrations between 0.06 and 5 μM and increasing splice-correction was observed at increasing concentrations of all compounds. We observed increased splice correction for ASOs 1b and 1c which have one and two 5boU units, respectively, compared to non-boronate-containing ASO 1a (Fig. 3C). Consistent with the CP50 values measured by CAPA, the most significant effects were observed at 60–200 nM concentrations, where 1b and 1c showed 2–3-fold greater splice-correcting activity than 1a. Interestingly, we observed no such increase for compound 1d which had three 5boU units. The suppressed splice-correcting activity of 1d may be due to its increased affinity towards biomolecules carrying multiple diols, such as complex glycans and RNA, once it reaches the cytosol.
Efficient delivery of therapeutic nucleic acids into cells presents a major challenge. In this work, we exploit the reversible interaction between boronic acid and cell surface glycans to increase cytosolic delivery of ASOs. We developed synthetic methods to install 5boU site-selectively within druglike oligonucleotides and used this chemistry to produce a panel of compounds each carrying an oligonucleotide sequence of interest, 5boU moiety, a linker, and a HaloTag substrate. In cell-based quantitative assays, boronated oligonucleotides exhibited improved cytosolic penetration by up to five-fold compared to non-boronate analogs. Remarkably, an ASO carrying three 5boU moieties was nearly as efficient as a small molecule. Our study constitutes the first report to successfully employ boronic acids to mediate oligonucleotide delivery inside human cells. It is worth noting that cytosolic penetration of the boronate ASOs is comparable to that of boronate proteins, as observed previously that RNAase A conjugated with 8–10 boronate units had 5-fold higher cell permeability compared to its non-boronate counterpart.8, 9 Our results further attest to the potential of boronic acids to promote cellular delivery of distinct biologics. We anticipate that further optimization of the boronate moiety and its placement within the bioactive oligonucleotide will furnish compounds with additionally enhanced activity. We also anticipate that high-resolution imaging experiments with fluorescently labeled boronate ASOs will lead to further insights into boronate-mediated interaction at the cell surface, mechanism of cellular uptake, kinetics of distribution within the cell, and interactions with cytosolic and nuclear components. Boronates represent a modular enhancement that could synergize with existing covalent and noncovalent delivery strategies for therapeutic nucleic acids.
Supplementary Material
Acknowledgments
We thank Tufts University, the University of Pittsburgh, the National Science Foundation (CHE-2204114 to K.I.) and the National Institutes of Health (GM127585 and GM148407 to J.A.K., R01GM130752 to K.I.) for financial support, Dr. D. Chakraborty and members of our laboratories for editing of the manuscript.
Footnotes
Conflicts of interest
There are no conflicts to declare
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.EWang F, Zuroske T and Watts JK, Nat. Rev. Drug Discov. 2020, 19, 441–442. [DOI] [PubMed] [Google Scholar]
- 2.Crooke ST, Witztum JL, Bennett CF and Baker BF, Cell Metab. 2018, 27, 714–739. [DOI] [PubMed] [Google Scholar]
- 3.Khvorova A and Watts JK, Nat. Biotech. 2017, 35, 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaczmarek JC, Kowalski PS and Anderson DG, Genome Med. 2017, 9, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannes L and Lucchino M, Nucleic Acid Ther. 2018, 28, 178–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Järver P, Coursindel T, Andaloussi SE, Godfrey C, Wood MJ and Gait MJ, Mol. Ther. Nucleic Acids 2012, 1, e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shukla RS, Qin B and Cheng K, Mol. Pharm. 2014, 11, 3395–3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ellis GA, Palte MJ and Raines RT, J. Am. Chem. Soc. 2012, 134, 3631–3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersen KA, Smith TP, Lomax JE and Raines RT, ACS Chem. Biol. 2016, 11, 319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsumoto A, Sato N, Kataoka K and Miyahara Y, J. Am. Chem. Soc. 2009, 131, 12022–12023. [DOI] [PubMed] [Google Scholar]
- 11.Das BC, Chokkalingam P, Masilamani P, Shukla S and Das S, Int. J. Mol. Sci. 2023, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang W, Fan H, Gao X, Gao S, Karnati VV, Ni W, Hooks WB, Carson J, Weston B and Wang B, Chem. Biol. 2004, 11, 439–448. [DOI] [PubMed] [Google Scholar]
- 13.Tan Y, Wu J, Song L, Zhang M, Hipolito CJ, Wu C, Wang S, Zhang Y and Yin Y, Int. J. Mol. Sci. 2021, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kavoosi S, Dey D and Islam K, Org. Lett. 2019, 21, 6614–6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deprey K, Batistatou N and Kritzer JA, Nucleic acids Res. 2020, 48, 7623–7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peraro L, Deprey KL, Moser MK, Zou Z, Ball HL, Levine B and Kritzer JA, J. Am. Chem. Soc. 2018, 140, 11360–11369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deprey K, Batistatou N, Debets MF, Godfrey J, VanderWall KB, Miles RR, Shehaj L, Guo J, Andreucci A, Kandasamy P, Lu G, Shimizu M, Vargeese C and Kritzer JA, ACS Chem. Biol. 2022, 17, 348–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thakur S, Sinhari A, Jain P and Jadhav HR, Front Pharmacol. 2022, 13, 1006304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kang SH, Cho MJ and Kole R, Biochem. 1998, 37, 6235–6239. [DOI] [PubMed] [Google Scholar]
- 20.Dominski Z and Kole R, Proceed. Natl. Acad. Sci. USA, 1993, 90, 8673–8677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Los GV, Encell LP, McDougall MG, Hartzell DD, Karassina N, Zimprich C, Wood MG, Learish R, Ohana RF, Urh M, Simpson D, Mendez J, Zimmerman K, Otto P, Vidugiris G, Zhu J, Darzins A, Klaubert DH, Bulleit RF and Wood KV, ACS Chem. Biol. 2008, 3, 373–382. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, So MK, Loening AM, Yao H, Gambhir SS and Rao J, Angew. Chem. Int. Ed. 2006, 45, 4936–4940. [DOI] [PubMed] [Google Scholar]
- 23.Andrei PG, Tet. Lett. 2011, 52, 434–437. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.