Figure 3.
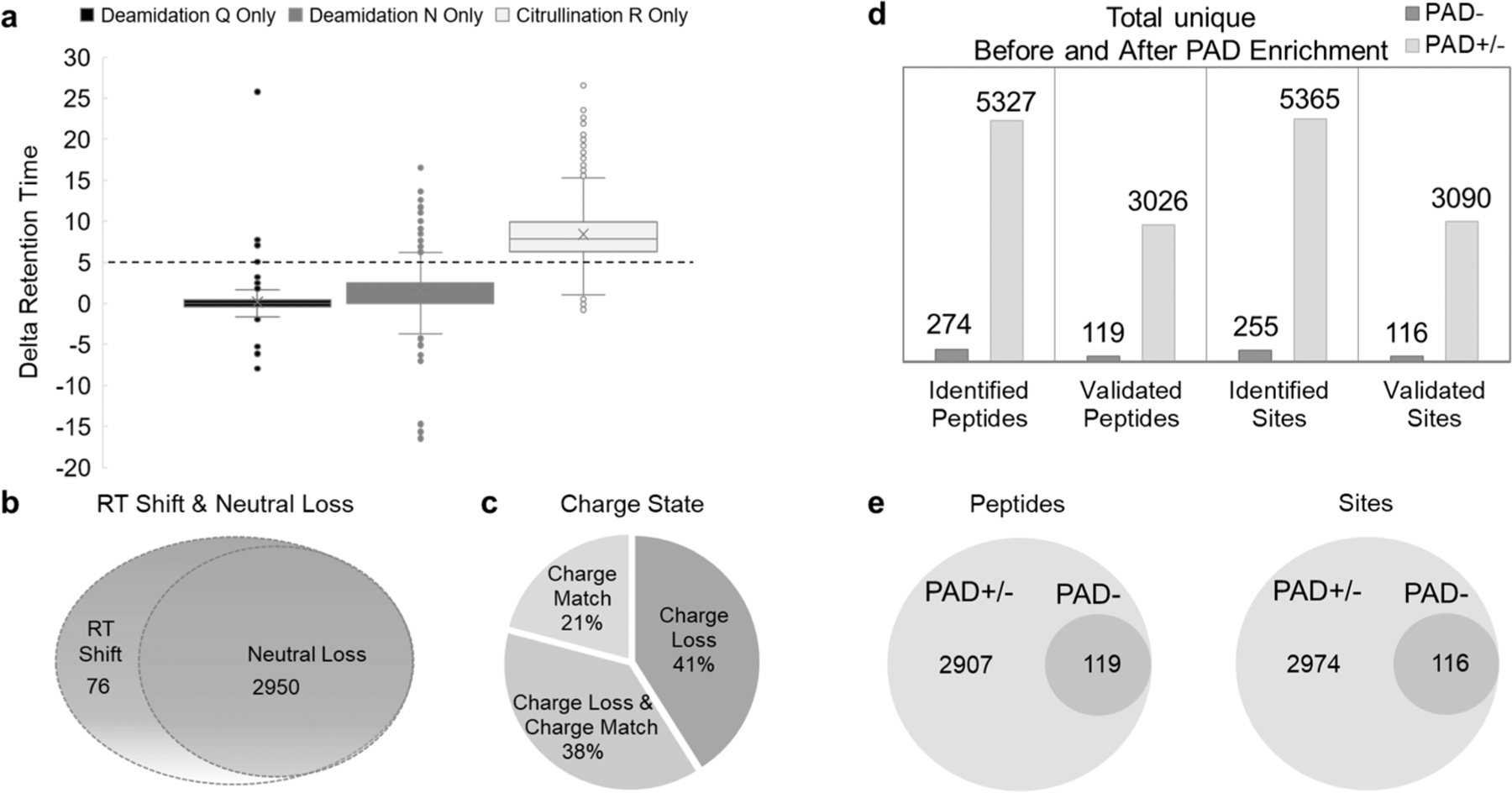
Physicochemical properties of citrullinated peptides. (a) Comparing the differences in ΔRT, modified to unmodified pairs for peptides containing deamidated amino acid asparagine (N), amino acid glutamine (Q), and citrullinated amino acid arginine (R). (b) Neutral loss distribution of 3026 validated peptides with a 5 min ΔRT shift. A peptide was considered to have a neutral loss if at least one product ion on MS/MS/MS spectra showed loss of HNCO (43 Da). (c) Charge distribution in 3026 validated peptides with a 5 min ΔRT shift. Prior to checking the charge state loss from peptides with a charge state higher than 3 observed with citrullination, we compared the charge status of all peptide match pairs. Only peptides that have match pairs were considered. (d) Bar graph summarizes the number of citrullinated peptides and sites identified with the enrichment method before and after validation. (e) Venn diagrams showing the distribution of identified citrullinated peptides and citrullinated residues across the hyper-citrullinated library (PAD±) and PAD− library.
