Figure 3. Conformal analysis for the homodimers and hetero-dimers of SEVI and Aβ.
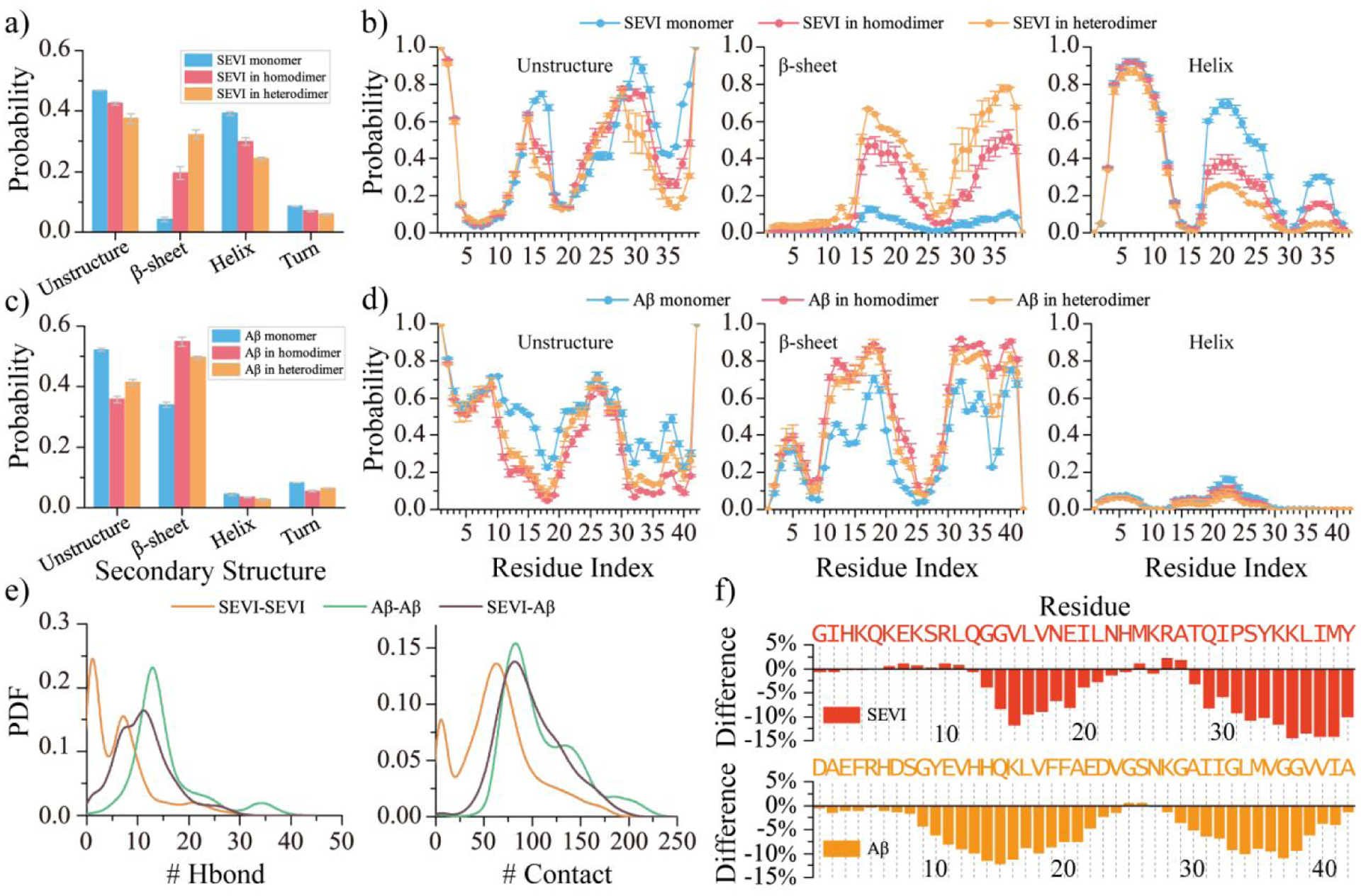
The average secondary content of SEVI a) and Aβ c) peptide in monomer, homo-dimer, and hetero-dimer. Probability of each residue from SEVI b) and Aβ d) adopting unstructured, β-sheet, and helix formations in monomer, homodimer, and heterodimer. The probability distribution of inter-peptide backbone hydrogen bonds and contacts in the SEVI and Aβ homo-dimer and hetero-dimer e). The change ratio of accessible surface area per residue of SEVI (upper) and Aβ (bottom) in the hetero-dimer compared to in the SEVI and Aβ isolated monomer f).
