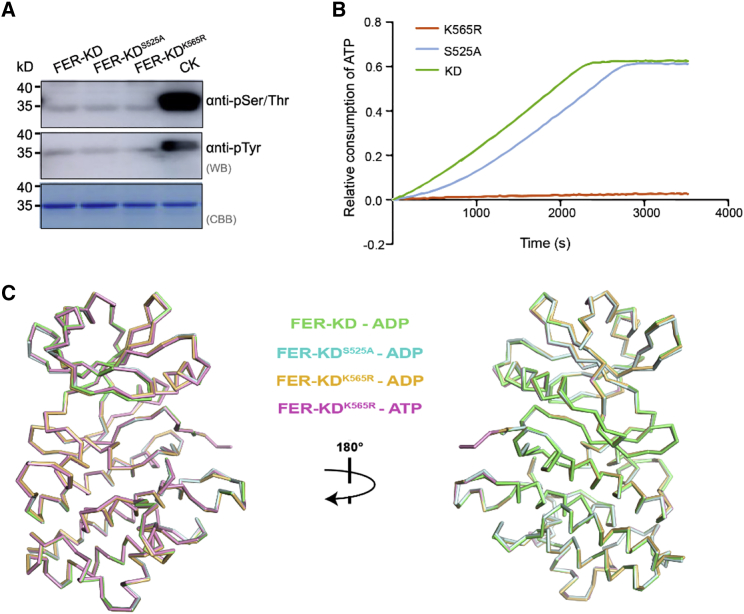
Figure 2.
Structural and biochemical characteristics of FER-KD and its mutants.
(A) Western blotting analysis of the phosphorylation state of FER-KD, FER-KDS525A, and FER-KDK565R. CK represents 1 mM FER-KD samples incubated with 1 mM ATP and 10 mM Mg2+ at 25°C for 30 min.
(B) ATPase activity of FER-KD, FER-KDS525A, and FER-KDK565R.
(C) Ribbon structural comparison of the FER-KD–ADP (green), FER-KDS525A–ADP (cyan), FER-KDK565R–ADP (orange), and FER-KDK565R–ATP (pink) complexes, which gives Cα RMSDs of 0.230, 0.280, and 0.187 Å, respectively.