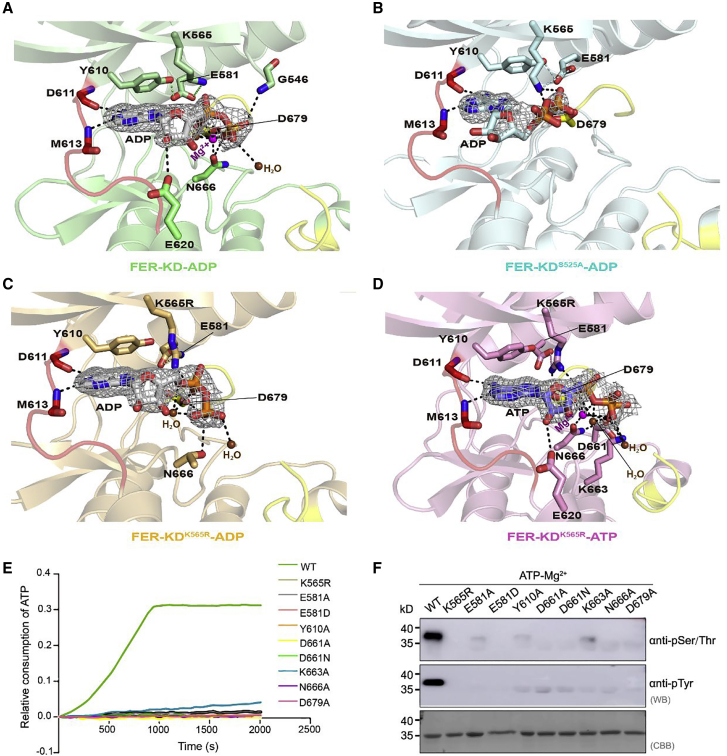
Figure 3.
Analyses of FER nucleotide-binding sites.
(A) Close-up view of the nucleotide-binding site of FER-KD–ADP (green). The E581–K565 salt bridge and E581–Y610 hydrogen bond are connected with green dotted lines, and other hydrogen bond contacts are connected with black dotted lines. The 2Fo-Fc electron density map, contoured at 1.6σ, is shown in gray mesh around the ADP molecule. The Mg2+ ions (purple) and water molecules (brown) are highlighted as spheres, whereas the ADP molecule and residues are represented as colored sticks.
(B–D) Close-up views of the FER-KDS525A–ADP (cyan), FER-KDK565R–ADP (orange), and FER-KDK565R–ATP (pink) complexes in the same orientation and color scheme as (A).
(E) ATPase activity of the nucleotide-binding-associated mutants.
(F) Western blotting analysis of the nucleotide-binding-associated mutants. Specifically, 1 mM unphosphorylated samples were incubated with 1 mM ATP and 10 mM Mg2+ for 30 min at 25°C.