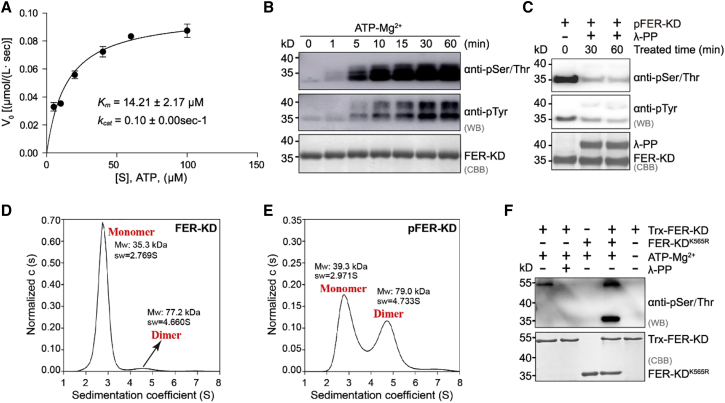
Figure 4.
FER-KD is autophosphorylated via an intermolecular mechanism.
(A) Measurement of kinetic parameters for FER-KD using the NADH-coupled microplate photometric assay. The reaction mixture contained 1 μM wild-type FER-KD and 5, 10, 20, 40, 60, or 100 mM ATP. The solid line represents the best-fit result according to the Michaelis‒Menten equation, with kcat and Km values of 0.10 ± 0.00 s−1 and 14.21 ± 2.17 mM, respectively.
(B) FER-KD time-course autophosphorylation assay. Specifically, 1 mM FER-KD samples were incubated with 1 mM ATP and 10 mM Mg2+ at 25°C for the indicated period.
(C) Phosphorylation states of FER-KD (pFER-KD) and lambda protein phosphatase-dephosphorylated pFER-KD. The reaction mixture contained 1 mM pFER-KD and 1 mM lambda protein phosphatase and was incubated for the indicated period.
(D) Sedimentation coefficient of FER-KD determined by sedimentation velocity analytical ultracentrifugation. The ratio relation of the protein oligomeric state of FER-KD protein is shown.
(E) Sedimentation coefficient of pFER-KD determined by sedimentation velocity analytical ultracentrifugation. The ratio relation of the protein oligomeric state of the pFER-KD protein is shown.
(F)Cis-/trans-autophosphorylation analyses of FER-KD by western blotting. The reaction mixture contained 1 mM Trx-FER-KD and 2 mM FER-KDK565R. Samples were incubated with 1 mM ATP and 10 mM Mg2+ at 25°C for 30 min.