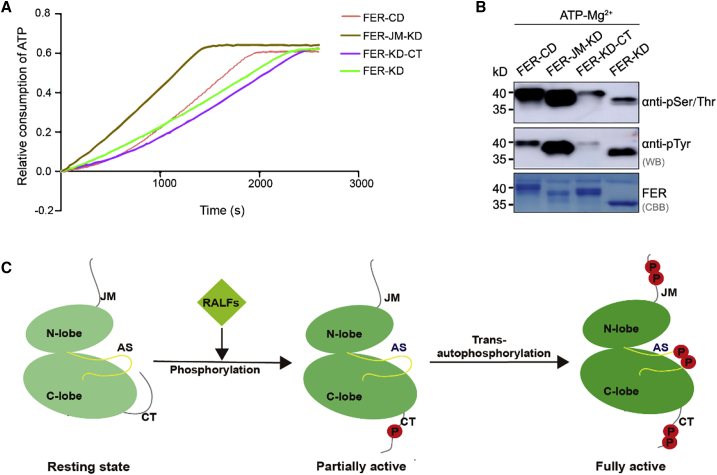
Figure 7.
Biochemical characteristics and model of the FER intracellular domain.
(A) ATPase activity of FER intracellular domain fragments.
(B) Western blot analysis of autophosphorylation by FER intracellular domain fragments.
(C) A model for stepwise activation of FER. In the absence of RALF ligands, FER kinase is unphosphorylated and autoinhibited by the CT. RALF ligand recognition induces conformational changes to FER, leading to phosphorylation of CT and thus promoting the release of its autoinhibition, placing the FER kinase in a partially active state. Further intermolecular autophosphorylation places FER in a fully activated state, and the activation segment is extended with phosphorylated sites to provide a platform for substrate binding.