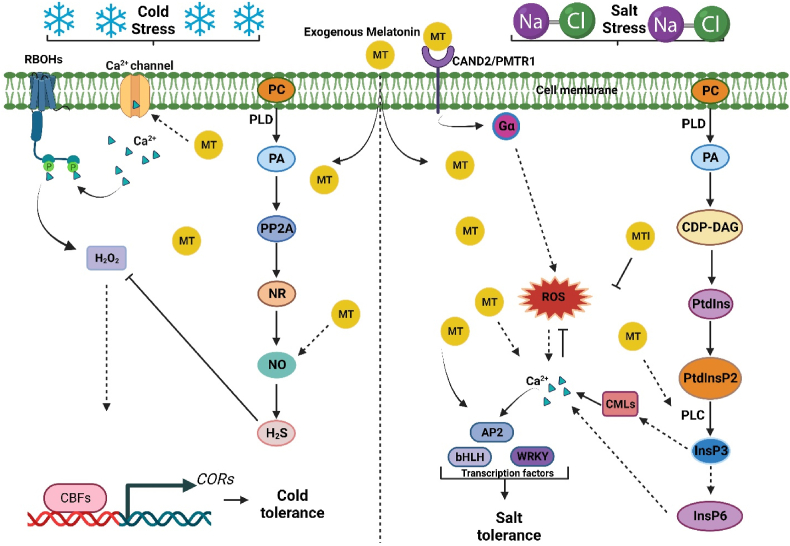
Fig. 3.
A regulatory model of interaction between melatonin (MT) and secondary messengers under abiotic stress. During cold stress, the membrane-bounded phospholipid phosphatidylcholine (PC) is degraded by phospholipase D (PLD) to produce phosphatidic acid (PA), which subsequently modulates the protein phosphatase 2A (PP2A), nitrate reductase (NR), nitric oxide (NO), and H2S. The exogenous application of MT triggers the Ca2+ channel to release Ca2+, which binds directly to the N-terminus of respiratory burst oxidase homologs (RBOHs), cold-induced Ca2+ activates respiratory burst oxidase activity to produce H2O2, thereby provoking Ca2+ transients in plant cells and causing downstream responses involving CBF-dependent pathways that promote cold tolerance. In case of salt stress, the PA is phosphorylated into cytidine diphosphate-diacylglycerol (CDP-DG), phosphatidylinositol (PtdIns), phosphatidylinositol bisphosphate (Ptd InsP2). The addition of MT up-regulates the phospholipase C (PLC), which hydrolysis the Ptd InsP2 into inositol 1,4,5 triphosphate (InsP3) and phosphorylated in subsequent steps to form phytic acid (InsP6), which triggers the release of Ca2+ in the cytoplasm. The MT receptor CAND2/PMTR1 also involves in the accumulation of Ca2+ by triggering G-protein G , which promotes ROS and Ca2+ production. Increased MT and Ca2+ influx up-regulates the transcription factors (AP2, bHLH, WRKY) to mitigate salt stress.