Fig. 4. Substrate scope of enantioselective borylation of C-P bond.
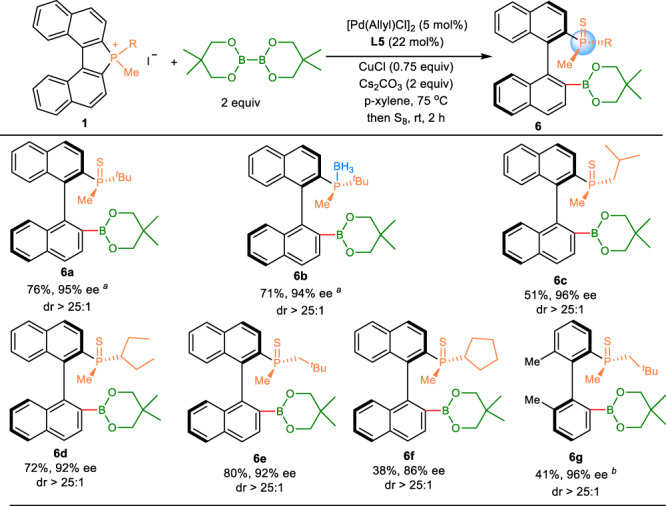
Reaction conditions: 1 (0.10 mmol), B2neop2 (2.0 equiv), [Pd(Allyl)Cl]2 (5 mol%), L5 (22 mol%), CuCl (0.75 equiv), Cs2CO3 (2.0 equiv), p-xylene (1.0 mL) for 12 h, then S8 (5 equiv) or BH3·SMe2 (2 equiv, 10 M in Me2S), unless otherwise stated. Isolated yields were reported. ee values of the major isomers are shown and determined by chiral HPLC analysis. dr values were determined by 1H NMR analysis of the crude reaction mixtures; athe reaction was conducted at 35 °C for 36 h; b0.10 mmol scale.
