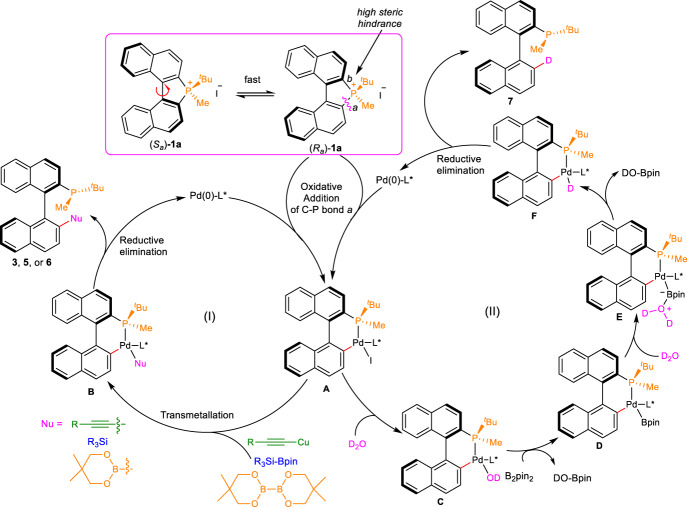
Fig. 7. Proposed reaction mechanism for the enantioselective cross-coupling and reduction of C–P bond.
Cycle I: Phosphonium salts (Sa)-1a and (Ra)-1a are in rapid equilibrium with each other via the rotation of the C–C single bond. The oxidative addition of Pd0 with substrate (R)-1a cleaves C–P bond a to form enantio-enriched biaryl intermediate A. Subsequent transmetallation of intermediate A with the terminal alkynes, R3Si-Bpin or diboron esters form the PdII species B. Reductive elimination of B furnishes the chiral phosphine product with concurrent regeneration of Pd0L* catalyst. Cycle II: The reaction of PdII intermediate A and water affords complex C. The transmetallation of complex C with the B2pin2 provides complex D. The reaction of complex D and water furnishes complex E. 1,4-D migration from E provides complex F. Reductive elimination of F delivers the desired product 7 with concurrent regeneration of Pd0L* catalyst.