Figure 1.
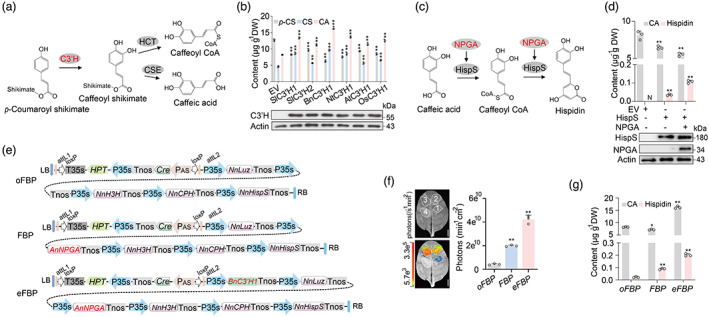
Mechanistic basis of C3′H and NPGA in enhancing luminescence emission. (a) Suggested mechanisms for C3′H involved in caffeoyl CoA and caffeic acid biosynthesis. C3′H, 4‐coumaroyl shikimate/quinate 3′‐hydroxylase; HCT, 4‐hydroxycinnamoyl CoA:shikimate/quinate hydroxycinnamoyltransferase; CSE caffeoyl shikimate esterase. (b) Mass spectra of caffeic acid produced by an in vitro enzyme assay using C3′H from different species. Three compounds of p‐Coumaroyl shikimate (p‐CS), caffeoyl shikimate (CS) and caffeic acid (CA) extracted from tobacco leaves transiently expressing indicated compounds were detected by standards, and empty vector (EV) was used as a control. C3′H‐Flag transient expression in tobacco leaves was determined by immunoblot analyses. (c) Proposed reaction catalysed by HispS and NPGA. HispS, hispidin synthase; NPGA, 4′‐phosphopantetheinyl transferase. (d) LC–MS/MS analysis of the content of Hispidin and Caffeic acid (CA) from injected tobacco leaves. HispS and NPGA transiently expressed in tobacco leaves were determined by immunoblot analyses. (e) The structure of binary constructs containing selectable marker HPT/marker‐excision Cre/loxP cassette (driven by PAS, anther‐specific promoter in tobacco). oFBP (1, original FBP), FBP (2) and eFBP (3, enhanced FBP) constructs are represented original bioluminescence, bioluminescence and enhanced bioluminescence, respectively. (f) Infiltration of WT tobacco leaves with oFBP, FBP and eFBP modules respectively. Bioluminescent intensity analysis from infiltrated leaves after 48 h. EV (4), empty vector. (g) Analysis of the content of hispidin and caffeic acid (CA) from infiltrated leaves after 48 h. Scale bars, 1 cm. Error bars indicate means ± SD (n = 3). Statistical significance was assessed using two‐tailed t‐tests (*P ≤ 0.05, **P ≤ 0.01).
