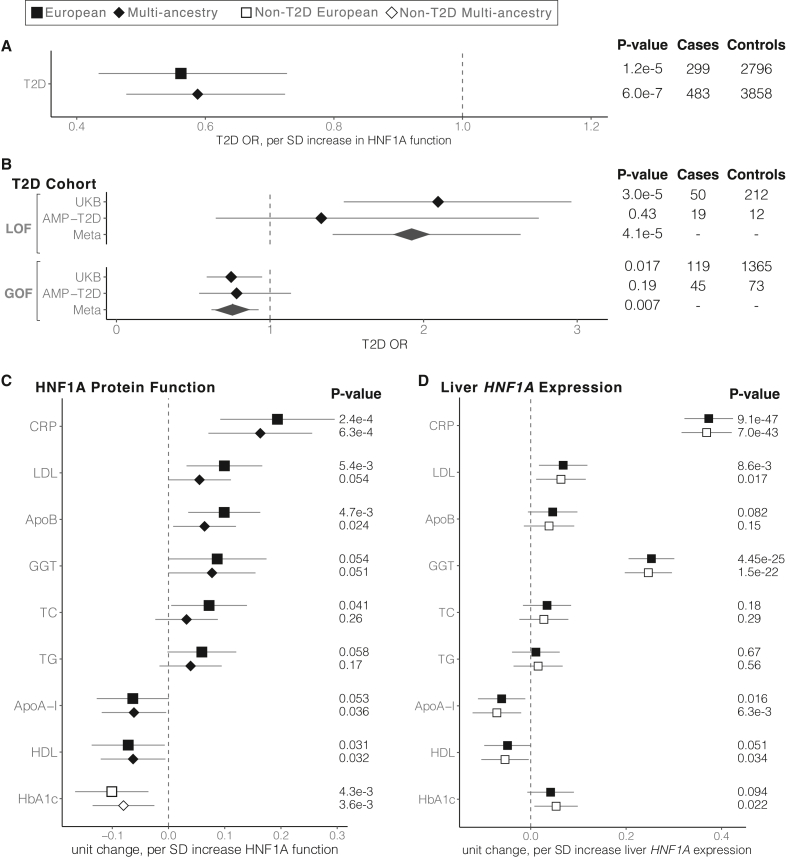
Figure 3.
HNF1A GOF variant carriers in the population are protected from T2D but show elevated levels of serum atherogenic risk factors
Shape and fill indicate the ancestry and T2D status, respectively, of individuals included in each analysis. All phenotypes are adjusted for age, age2, sex, and the first 10 principal components of ancestry. T2D, type 2 diabetes; SD, standard deviation; LOF, loss of function; GOF, gain of function; CRP, C-reactive protein; LDL, low-density lipoprotein cholesterol; ApoB, apolipoprotein B; GGT, gamma-glutamyl transferase; TC, total cholesterol; TG, triglycerides; ApoA-I, apolipoprotein A-I; HDL, high-density lipoprotein cholesterol; HbA1c, glycated hemoglobin.
(A) Association of HNF1A function score and T2D risk using logistic regression in the UKB HNF1A rare protein-coding variant carriers identified among the 454,756 exome-sequenced individuals. Odds ratios and the 95% confidence interval are shown.
(B) Association of LOF and GOF HNF1A variants with T2D in the UKB and AMP-T2D (n = 20,791 cases, 24,440 controls) using logistic regression. Odds ratios and the 95% confidence interval are shown, and fixed effects inverse-variance meta-analysis was used to combine the results.
(C) Association of functional HNF1A variants with cardiovascular disease risk factors in the UKB rare variant carriers (European n = 3,089, multi-ancestry n = 4,302, linear mixed-effects regression). Effect size and the 95% confidence interval are shown for each phenotype.
(D) Association of liver-specific predicted HNF1A gene expression with disease risk factors tested in (C) among all UKB European genotyped individuals (n = 407,227, filled squares) and non-T2D European genotyped individuals (n = 376,043, open squares).