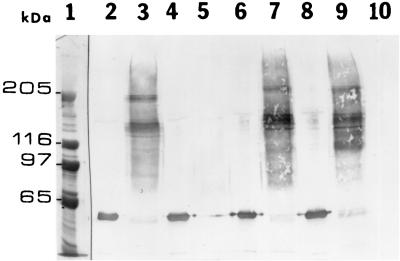
FIG. 3.
Binding and oligomerization of toxins in M. sexta BBMV. BBMV (20 μg of protein) were incubated with 60 ng of toxin at 30°C for 1 h. The vesicles were centrifuged, washed, and extracted as described in Materials and Methods. The extracts were subjected to electrophoresis (SDS–6% polyacrylamide gels) and western blotting, and the immunoblots were treated with rabbit anti-Cry1Ac1 antibody and then an anti-rabbit antibody conjugated with alkaline phosphatase. Lane 1, molecular mass standards; lane 2, wild-type toxin; lane 3, wild-type toxin extracted from BBMV; lane 4, mutant toxin A164P; lane 5, mutant toxin A164P extracted from BBMV; lane 6, mutant toxin Q133R; lane 7, mutant toxin Q133R extracted from BBMV; lane 8, mutant toxin I132S; lane 9, mutant toxin I132S extracted from BBMV; lane 10, BBMV. All other nontoxic helix α4 mutants also oligomerized in the membrane (data not shown).