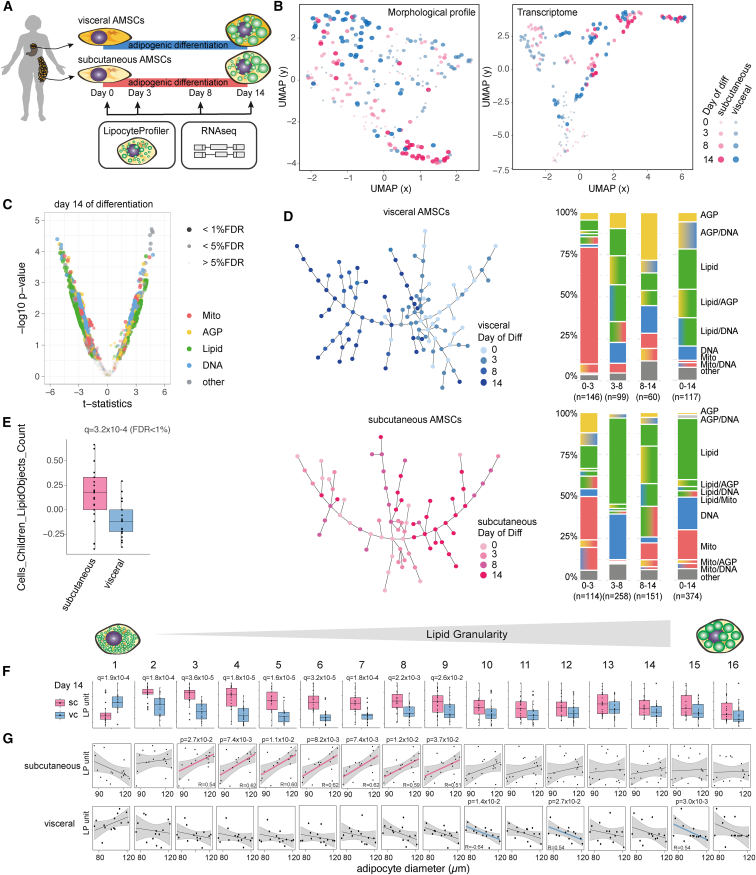
Figure 2.
LipocyteProfiler identifies distinct depot-specific morphological and cellular signatures associated with differentiation trajectories in both visceral and subcutaneous AMSCs
(A) Human AMSCs isolated from subcutaneous and visceral adipose depots were differentiated for 14 days, and LipocyteProfiler and RNA-seq profiling were performed throughout adipocyte differentiation (days 0, 3, 8, and 14).
(B) LipocyteProfiler and transcriptome profiles show time-course-specific signatures revealing a differentiation trajectory, but only LipocyteProfiler additionally resolves adipose-depot-specific signatures.
(C) Subcutaneous and visceral AMSCs at terminal differentiation (day 14) have distinct morphological and cellular profiles with differences that are spread across all channels. See also Figure S2C (volcano plot reporting the −log10 p value and the effect comparing subcutaneous and visceral adipocytes, t test).
(D) Sample progression discovery analysis (SPD). Proportions of subgroups of features characterizing differentiation differ between subcutaneous and visceral adipocytes and dynamically change over the course of differentiation. In both depots, Mito features drive differentiation predominantly in the early phase of differentiation (days 0–3) whereas Lipid features predominate in the terminal phases (days 8–14). See also Figure S2D for SPD of hWAT and SGBS.
(E) The number of lipid droplets is higher in subcutaneous AMSCs than in visceral AMSCs at terminal differentiation. y axis shows LP units (normalized LP values across eight batches, see STAR Methods).
(F) Mature subcutaneous AMSCs have larger intracellular lipid droplets compared with visceral AMSCs at day 14 of differentiation (Lipid Granularity). y axis shows autoscaled LP units (normalized LP values across eight batches, see STAR Methods).
(G) Lipid Granularity from subcutaneous AMSCs at day 14 of differentiation correlates positively with floating mature adipocyte diameter but shows an inverse relationship for visceral adipose tissue, suggesting distinct cellular mechanisms that lead to adipose tissue hypertrophy in these two depots. y axis shows autoscaled LP units (normalized LP values across eight batches; x axis, histology adipocytes diameter [μm], see STAR Methods).