Figure 6.
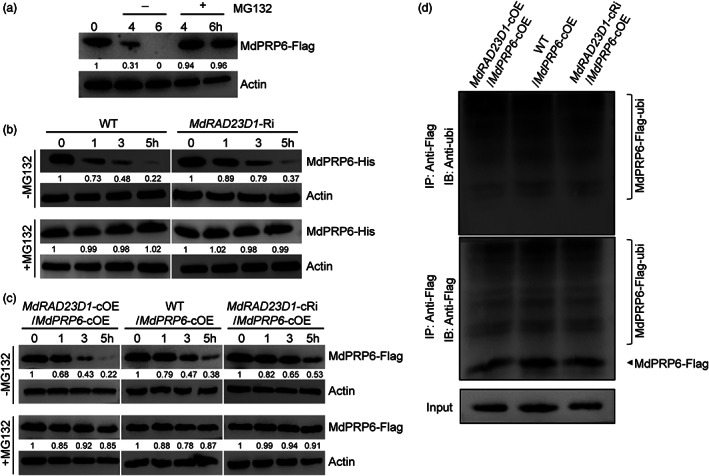
MdRAD23D1 promotes the degradation of MdPRP6. (a) Effects of MG132 on MdPRP6 degradation. The transgenic apple calli expressed 35S::MdPRP6‐Flag were treated with 75 μm CHX with (+) or without (−) 50 μm MG132 for the indicated time, and the amount of MdPRP6‐Flag was determined using anti‐Flag antibody. (b) Cell‐free degradation assays in apple plants. The Escherichia coli–expressed MdPRP6‐His protein was incubated with the protein extracts from WT or MdRAD23D1‐Ri apple plants with or without MG132, and then immunoblotting was performed using anti‐His antibody at the indicated time points. (c) MdRAD23D1 affects the degradation of MdPRP6 in vivo. Total proteins separately were extracted from the WT/MdPRP6‐cOE, MdRAD23D1‐cOE/MdPRP6‐cOE, and MdRAD23D1‐cRi/MdPRP6‐cOE transgenic apple calli after treated with 75 μm CHX with or without MG132. The immunoblotting was performed with anti‐Flag antibody. (d) Ubiquitination of MdPRP6 in vivo. The total proteins were separately extracted from the same three calli in (c) and immunoprecipitated using anti‐Flag magnetic beads. The MdPRP6‐Flag protein was detected with anti‐ubi antibody and anti‐Flag antibody. WT in (b), wild type, here we used GL‐3 apple (Malus domestica), which was also used as explants in generating transgenic apple plants; MdRAD23D1‐Ri, transgenic apple plants with suppressed expression of MdRAD23D1 via RNA interference; MdRAD23D1‐cOE/MdPRP6‐cOE, transgenic apple calli co‐expressed 35S::MdRAD23D1‐HA and 35S::MdPRP6‐Flag; MdRAD23D1‐cRi/MdPRP6‐cOE, transgenic apple calli with suppressed expression of MdRAD23D1 via RNA‐interference, and co‐expressed with 35S::MdPRP6‐Flag; WT/MdPRP6‐cOE, transgenic apple calli expressed 35S::MdPRP6‐Flag. All the transgenic apple calli was generated using ‘Orin’ calli (M. domestica). MdActin was used as the loading control (a–c) or input control (d). The amounts of proteins were quantified using an Ultra‐sensitive multifunctional imager (Uvitec), and the protein levels at 0 h were set to 1.
