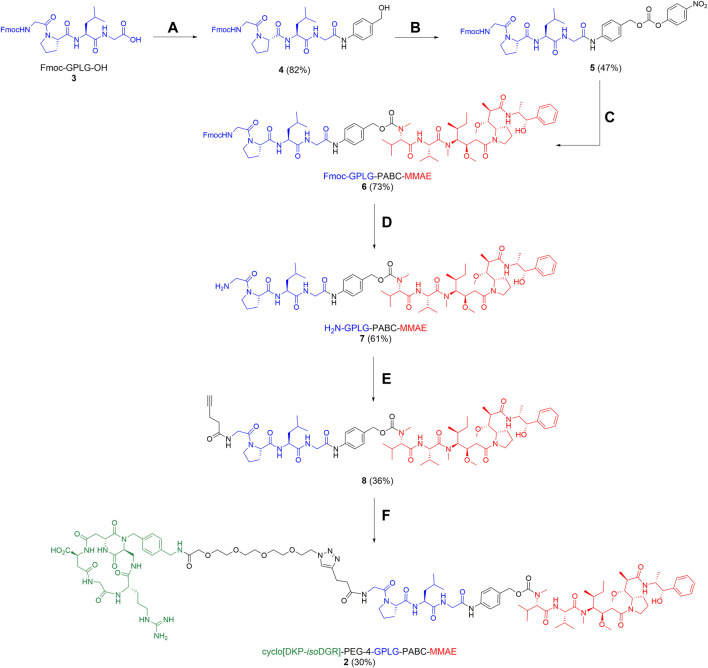
SCHEME 2.
Synthesis of conjugate 2. Reagents and conditions: (A) PAB-OH, EEDQ, DCM/MeOH, rt, overnight; (B) 4-nitrophenylchloroformate, pyridine, THF, rt, 6 h; (C) MMAE, HOAt, DIPEA, DMF, rt, 46 h; (D) piperidine, DMF, rt, 2 h; (E) N-(4-pentynoloxy)succinimide, DIPEA, DMF, rt, 2 h; (F) cyclo[DKP-isoDGR]-PEG4-N3 9, CuSO4∙5H2O, sodium ascorbate, DMF/H2O (1:1), rt, 25 h.