Abstract
Essential tremor (ET) is one of the most common neurological disorders and can be highly disabling. In recent years, studies on the clinical perspectives and pathophysiology have advanced our understanding of ET. Specifically, clinical heterogeneity of ET, with co-existence of tremor and other neurological features such as dystonia, ataxia, and cognitive dysfunction, has been identified. The cerebellum has been found to be the key brain region for tremor generation, and structural alterations of the cerebellum have been extensively studied in ET. Finally, four main ET pathophysiologies have been proposed: 1) environmental exposures to β-carboline alkaloids and the consequent olivocerebellar hyper-excitation, 2) cerebellar GABA deficiency, 3) climbing fiber synaptic pathology with related cerebellar oscillatory activity, 4) extra-cerebellar oscillatory activity. While these four theories are not mutually exclusive, they can represent distinctive ET subtypes, indicating multiple types of abnormal brain circuitry can lead to action tremor.
Keywords: Essential tremor, Cerebellum, Oscillation, Physiology, Electroencephalogram, Tremor
1. Introduction
Essential tremor (ET) is one of the most common neurological disorders, and many people are disabled from ET because current therapeutic options are rather limited. The major challenge of therapy development for ET is the obscured ET pathophysiology for tremor generation. In the past decade, the advances in ET genetics, environmental factors, animal models, pathology, and physiology converge on the important role of the cerebellum and the associated cerebello-thalamo-cortical loop. These studies not only identify important therapeutic targets for ET but also further our understanding how the dysfunctional cerebellum can drive this unique type of rhythmic, involuntary movements (i.e. tremor). In this review, we will briefly review 1) clinical aspects of ET, 2) current tools to measure and study brain activity related to ET, 3) theoretical ET pathophysiology, which will shed lights on novel therapeutic development.
2. Clinical perspectives of ET
ET is highly prevalent and occurs in people with different ethnic background. The prevalence of ET is estimated to be 0.32–1.33% of the worldwide population [1]. The prevalence of ET increases by 74% for every decade increase in age, reaching >20% in the elderly in some studies [2]. The prevalence of ET also varies in different geographic regions [2]. The age of onset for ET has a bimodal distribution [3], and ET thus can be divided into young onset vs. old onset ET. Young onset ET cases are more likely to have a family history of tremor [4], whereas old onset ET cases often have faster disease progression [5]. Despite the distinct differences in the natural history and possibly certain physiological features [6], young onset ET and old onset ET seem to reach the same pathological endpoint since no distinguishing pathological features in the postmortem examination between these two groups were found [7]. Finally, there is no convincing evidence that age of onset can predict pharmacological responses in ET, except that young onset ET may be more responsive to alcohol [4].
In 2018 Movement Disorders Society Task Force proposed the ET diagnosis as isolated tremor syndrome of bilateral, upper-limb action tremor for at least 3 years, with or without tremor in other body locations, such as in head, voice, or lower limbs, and the absence of other neurological signs, such as dystonia, ataxia, and parkinsonism [8]. While these criteria provide useful research guidelines, it is increasingly recognized that 54–85% patients who otherwise fit the ET diagnostic criteria have above-mentioned neurological signs (e.g., dystonia, ataxia, and parkinsonism) [9,10], and some experts categorize this group of patients with a controversial term: ET-plus. There is no doubt that ET can have heterogeneous clinical presentations but whether ET-plus is a useful concept to describe the heterogeneity of ET is still under heated debate [11–13]. A recent neuropathological study found no differences in cerebellar pathology in ET cases with and without these associated neurological signs [14], and another clinical study found that the presence of these additional neurological signs in ET patients correlates with disease duration, indicating these signs could be part of the natural evolution of the disease [15].
ET is a progressive disorder. Tremor becomes larger in amplitude and thus disabling over the course of disease [16], interfering with activity of daily living. To date, no disease modifying therapies exist for ET. On the other hand, several pharmacological and surgical interventions for symptomatic therapies are available. The first line therapies for ET are primidone and propranolol [17]. However, only approximately 50% ET patients respond to first line therapies, and the effects on tremor suppression is only about 50% [18]. Topiramate [19] and benzodiazepines [17] can be the second line therapies for ET. Many of these pharmacological agents may cause side effects, such as sedation and cognitive impairment, especially in the elderly population. Botulinum toxin injections may dampen the tremor in ET patients but could sometimes come with the cost of hand muscle weakness [20]. Surgical options can be highly effective to treat ET. Deep brain stimulations targeting ventral intermediate nucleus of the thalamus, which receives the cerebellar outflow, can dramatically suppress tremor [21]. Notably, a subset of ET patients receiving deep brain stimulation experiences “habituation”, which means gradual return of tremor requiring higher settings of deep brain stimulation [22]. A higher setting for the deep brain stimulation may result in non-specific stimulations of the neighbor brain regions, causing side effects of dysarthria and/or abnormal sensation [22–24]. Alternatively, targeted lesioning in the thalamus by focused ultrasound can be another method to achieve tremor suppression [25]. Similarly, some patients may experience a gradual return of tremor during 5-year follow up [26]. The habituation phenomenon is hypothesized to be related to the re-organization of brain circuitry of tremor with chronic suppression of tremor-related oscillations [22,27]. In summary, despite some available pharmacological and surgical options for symptomatic therapies, many ET patients still have unsatisfactory responses and experience side effects. Therefore, targeted therapies addressing the source of tremor generation hold promise for novel therapy development for ET.
3. Objective tremor measurements
A major challenge for ET therapy development and research comes from the facts that the diagnosis of ET solely relies on clinical evaluation and neurological examination, and there is a requirement of 3-year disease duration [8]. Therefore, development of objective measures can further aid the diagnosis as well as understand the pathophysiology of ET. Fortunately, tremor is among the most trackable involuntary movements with defined frequency, amplitude, and phase, which are ideal for objective measurements.
Physiological tools have been applied to measure and to quantify rhythmic movements of the limbs (i.e. tremor). Surface electromyography (EMG) and accelerometers are the key tools [28–31]. Surface EMG measures integrated voltage changes in the surface of the muscles whereas accelerometers measure targeted limb oscillatory movements. In the physiological condition, the skeletal muscular “mechanical” system has its intrinsic oscillations that can be detected by the accelerometer, but typically these subthreshold oscillatory activity does not lead to rhythmic muscular contractions [29], unless the oscillatory activity is amplified by stress or caffeine intake, which is called enhanced physiological tremor. Enhanced physiological tremor usually at the frequency of 4–12 Hz [30,32,33], is considered having a peripheral origin (i.e. from the muscles) rather than coming from the oscillatory activity in the central nervous system. Therefore, weight-loading on the limb of enhanced physiological tremor, which increases the inertia of the mechanical system, can reduce tremor frequency [30,32,33]. Similar to enhanced physiological tremor, ET patients also have action tremor at the frequency of 4–12 Hz, detected by both surface EMG and accelerometers [34]. However, tremor in ET does not change frequency with weight-loading, providing a strong evidence that peripheral mechanical source is not the origin of ET [28,33,35]. Since physiological tremor and ET can co-exist, weight-loading can shift the frequency of the mechanical part of tremor but not alter tremor coming from the central nervous system, allowing to dissect different components of tremor [28,35]. Besides enhanced physiological tremor, dystonic tremor and parkinsonian tremor are major differential diagnoses of ET. Compared to ET, dystonic tremor shows greater instability in terms of tremor frequencies and amplitudes, and has task-specific changes of coherence between agonist and antagonist muscle pairs [36]. While parkinsonian tremor occurs predominantly at rest and ET is characterized by action tremor, it is increasingly recognized that postural tremor commonly occurs in Parkinson’s disease [37]. To add to the complexity, postural tremor in Parkinson’s disease can be further divided into re-emerging postural tremor and pure postural tremor, and the latter could phenomenologically resemble tremor in ET [37]. These could sometimes raise diagnostic dilemma whether patients first developed ET and then later on Parkinson’s disease or these types of postural tremor are early signs of Parkinson’s disease. Another important distinction between ET and parkinsonian tremor is in the tremor kinematics. Specifically, tremor in ET, when compared to parkinsonian tremor, exhibits greater sinusoidal stability captured by accelerometers and can be quantified by the tremor stability index [38].
4. Mapping brain circuits of ET
Since findings from EMG and accelerometers suggest the non-peripheral, or central origin of tremor generator in ET, investigators have been using tremor frequency as a way to identify oscillatory activity and to find the source of tremor. Specifically, electroencephalogram (EEG) and magnetoencephalogram (MEG) were implemented to study the functional connectivity and to perform frequency analysis in ET patients. The major findings of these techniques indicate the role of the cerebello-thalamo-cortical loop in ET [39–42]. These physiological studies are consistent with clinical observations that discrete strokes within this loop can disrupt tremor in ET patients, and the locations of strokes include primary motor cortex, pontine nuclei, cerebellum, dentate nucleus, posterior part of ventrolateral nucleus and ventral intermedius nucleus of the thalamus [43–46]. In addition, the combination of EEG and EMG to study cortico-muscular coherence in ET patients reveals the co-involvement of primary motor cortex and the cerebellum [47,48]. Since the converging evidence indicates the critical role of the cerebellum in ET, a recent study showed enhanced cerebellar oscillatory activity can be directly recorded by cerebellar EEG in ET patients [41]. Not only the cerebello-thalamo-cortical loop is important for ET, such loop also plays a role in parkinsonian tremor. A high density EEG study suggests that oscillatory brain activity in ET and parkinsonian tremor flows mainly from the cerebellum to the sensorimotor cortex whereas the oscillatory activity flows in the opposite direction in mimicked tremor [49]. These data demonstrate that the brain circuitry overlaps between ET and parkinsonian tremor.
In addition to the neurophysiology techniques, neuroimaging also provides excellent spatial resolution to map out the brain circuit of ET. Specifically, functional magnetic resonance imaging (fMRI) studies showed the involvement of the cerebello-thalamo-cortical circuit, with converged evidence showing increased cerebellar activities during motor events [43,50–54], or cerebellar participation in resting-state functional connectivity in ET patients [55–62]. Another fMRI study documented the association between the tremulous limb and the contralateral primary motor cortex, while bilateral cerebellar cortices are simultaneously involved. The fMRI activation pattern in ET is in sharp contrast to the mimicked tremor by volitional rhythmic movements in healthy controls, which involves only the ipsilateral cerebellum [52].
Finally, non-invasive brain stimulations, such as transcranial magnetic stimulation (TMS) and transcranial alternative current stimulation (tACS), to the relevant brain regions can provide stronger evidence of the causative involvement of neuronal structures in tremor. Single- or paired- pulse TMS on the supplementary motor cortex, primary cortex and the cerebellum can reset the phase of tremor [63,64]. Paired-pulse TMS also helps to distinguish dystonic tremor from tremor with dystonia [36]. Based on the accelerometer-detected hand tremor in ET patients, phase-locked tACS stimulation to the cerebellum can modulate tremor amplitudes [65]. Phase-locked deep brain stimulations in the thalamus can also tune tremor amplitude or entrained the tremor frequency in ET patients [66–68]. These studies suggest that supplementary motor cortex, primary motor cortex, thalamus and the cerebellum contribute to the online generation of tremor in ET.
5. Cerebellar pathology of ET
The above-mentioned studies have identified the cerebellum as a critical region for ET, the next question is what’s happening in the ET cerebellum? To answer this, large efforts have been invested to understand the structural alterations in the postmortem ET cerebellum. As the results, several pathological features, centered around Purkinje cells, have been identified. ET cerebellum has modest loss of Purkinje cells, swelling of Purkinje cell axons, called torpedoes [69], and the displacement of Purkinje cell bodies away from the Purkinje cell layer [70]. These pathological changes are commonly regarded as consequences of cerebellar damages. In addition to torpedoes, which can be easily observed in the thin sections, more sophisticated thick section analysis of the ET cerebellum revealed that Purkinje cell axons undergo a variety of re-organization, such as recurrent collaterals, thickening, and branching [71]. In the dendritic compartment, Purkinje cells in the ET cerebellum also have dendritic swelling [72] and reduced dendritic spines [73], indicating that both dendritic and axonal compartments of Purkinje cells are affected. A recent study showed that Purkinje cell axonal swelling can enhance action potential fidelity [74], which may have implications for tremor.
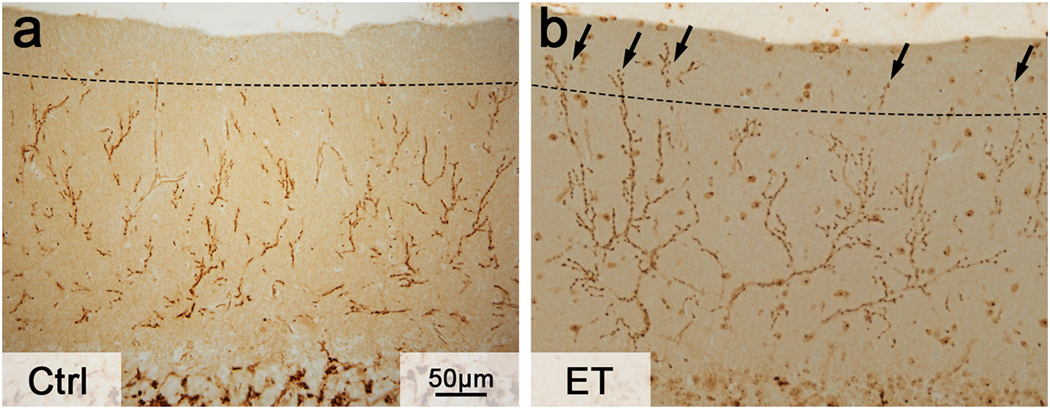
Not only Purkinje cell damages are evident in the ET cerebellum, other neighboring neuronal cell types may also be affected. In ET, basket cells develop more dense and elongated plexus surrounding Purkinje cell axonal initial segment [75,76] whereas climbing fibers form abnormal synaptic connections with Purkinje cells in the parallel fiber synaptic territory (Fig. 1) [77,78]. These detailed pathological analysis help us to gain insight into the structural alterations in the ET cerebellum.
Fig. 1.
Climbing fiber synaptic pathology in ET. (A) In a control subject, climbing fiber synapses, as visualized by the vesicular glutamate transporter type 2 immunohistochemistry, usually do not extend to the outer 20% of the molecular layer (dash line), which is the parallel fiber synaptic territory. (B) In a ET patient, climbing fiber synapses abnormally extend to the parallel fiber synaptic territory (arrows).
Many of the observed morphological changes in the ET cerebellum reflect degenerative changes in Purkinje cells. However, ET has distinct clinical features very different from other putative cerebellar degenerative disorders. To investigate the specificity, large pathological studies have been performed to compare ET to ataxic disorders, such as spinocerebellar ataxia and multiple system atrophy. Among all the pathological features, climbing fiber synaptic pathology appears to be the most specific change to ET. ET cerebellum has climbing fiber synaptic territory expansion, extending into the parallel fiber synaptic territory, whereas ataxic cerebellum has regression of climbing fiber synaptic territory [79,80]. Interestingly, climbing fiber synaptic pathology occurs across ET patients with different clinical characteristics [81], indicating that this pathology may be related to the core clinical feature of ET (i.e., tremor). Not only climbing fibers extend to the parallel fiber synaptic territory in ET, climbing fibers also form lateral crossing to innervate multiple Purkinje cells, and this particular pathological feature correlates with tremor severity [82]. Since the source of climbing fibers is the inferior olive, which has intrinsic oscillatory property, the distal extension and multiple Purkinje cell innervation of climbing fibers can possibly influence the pacemaking activity of Purkinje cells and cerebellar physiology, leading to tremor.
While morphological changes have been observed in the ET cerebellar cortex, the next question is whether the downstream deep cerebellar nuclei are also affected. A study found that there is no change in the dentate nucleus neuronal density in ET [83], indicating no neuronal loss in this region. Since deep cerebellar nuclear neurons receive GABAergic inputs from Purkinje cells, detailed postmortem analysis of GABAA and GABAB receptor binding found that the dentate nucleus of ET patients has reduction of both types of receptors [84].
In addition to the morphological alterations, gene expression analysis of the ET cerebellum revealed a variety of molecular pathways that can underlie the structural changes. These pathways are 1) calcium signaling and synaptic transmission, 2) axon guidance, 3) microtubule motor activity, 4) endoplasmic reticulum to Golgi transport [85]. These molecular studies will likely yield important therapeutic targets for novel ET therapy development.
6. Pathophysiology of ET
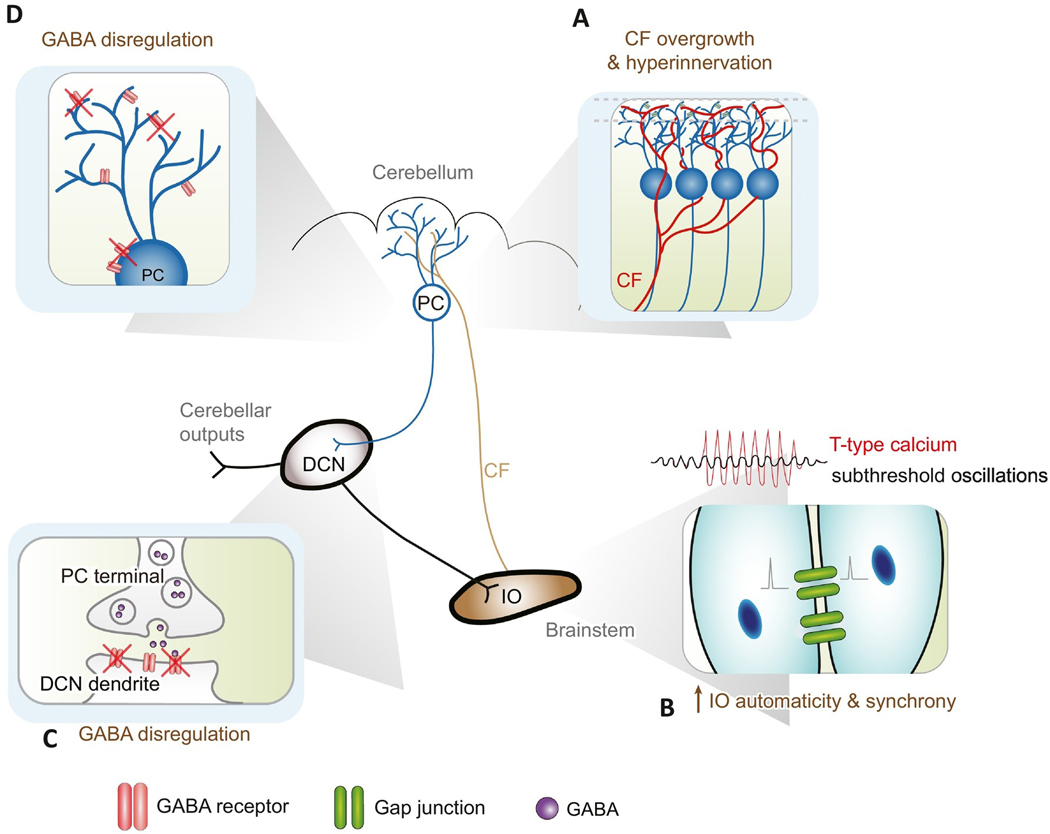
The recent advances in ET genetics, environmental factors, human pathology, and animal models have dramatically changed our knowledge of ET pathophysiology in the past decade. Here, we will review four major theories on ET pathophysiology and the current understanding how the dysfunctional cerebellar circuit can contribute to tremor (Fig. 2).
Fig. 2.
Summary of ET pathophysiology. (A) Climbing fiber overgrowth and hyperinnervation. Reduction of GluRδ2 protein in the Purkinje cells causes climbing fiber synaptic pruning deficits, which lead to hypersynchrony and excessive oscillations in Purkinje cells. (B) Increased inferior olive automaticity and synchrony. β-carboline alkaloids, such as harmaline, lead to augmented T-type calcium channel function and potentially increased inferior olive synchrony via gap junctions. (C & D) GABA dysregulation. GABA receptor knockout in Purkinje cells and reduced GABA receptors in the deep cerebellar nucleus can lead to abnormal oscillations in the circuitry. DCN: deep cerebellar nucleus; IO: inferior olive; PC: Purkinje cell.
6.1. β-carboline alkaloid exposures and related tremor
While 62.5% ET patients have a family history tremor [86], suggesting a strong genetic component, ET genetics have been a challenging area of research. The cause of ET is likely to come from complex genetic and environmental interactions. Several environmental factors have been identified to play roles in ET, such as pesticide, heavy metals, ethanol, and β-carboline alkaloids [87]. β-carboline alkaloids are a class of compounds enriched in meat. Harmane, a major β-carboline alkaloid, is elevated in the blood of ET patients compared to that of controls [88–90]. In addition, harmane levels are also elevated in the postmortem ET cerebellum [91], further supporting a role of β-carboline alkaloids in ET.
Another well-known β-carboline alkaloid is harmaline, and harmaline can induce acute action tremor in rodents, cats, and primates. Therefore, harmaline-induced animal tremor is considered as a classical model of ET [92]. Harmaline-induced tremor can be dampened by ET medications, propranolol, primidone, and alcohol [93]. Harmaline induces tremor by enhancing the automaticity in the inferior olivary neurons via a mixture of proposed pharmacological actions related to NMDA channels [94], T-type calcium channels [95], and possibly gap junctions between inferior olivary neurons for electrical coupling [96]. Gap junctions in the inferior olivary neurons serve as electrical synapses to synchronize their neuronal activity [97,98]. In addition, such gap junctions are controlled by the GABAergic inputs from the deep cerebellar nuclei, thus, forming a close loop control within the olivo- cerebellar cortex-deep cerebellar nuclei, which is capable of generating oscillatory activity [99,100]. Interestingly, eliminating the gap junctions in the inferior olive reduces the synchrony between inferior olivary neurons but does not stop harmaline-induced tremor, suggesting that oscillations at the circuitry level beyond intra-inferior-olivary synchrony are also important for tremor generation [101].
Among the mechanism of harmaline-induced tremor, T-type calcium channel is of particular interests. A recent study has identified a ET family with the mutation in the gene that encodes T-type calcium channel, CACNA1G [102]. T-type calcium channel is important for the pacemaking activity in both inferior olivary neurons [103] and Purkinje cells [104]. Interestingly, T-type calcium channel antagonists can suppress tremor in harmaline-induced rodent tremor [105], further supporting a potential for tremor therapy. A recent double-blinded, placebo-controlled ET clinical trial on T-type calcium channel modulator, CX-8998, showed that such agent can reduce the severity scores of site investigator-rated The Essential Tremor Rating Assessment Scale (TETRAS) and TETRAS-activity daily living. However, the primary end point, the centrally video-rated TETRAS, did not show any improvement [106]. It is unknown why centrally-video rating and site investigator rating have such differences. Nonetheless, T-type calcium channels still remain targets of interests for ET therapy development, and further investigations are needed.
Harmaline-induced tremor depends on the olivocerebellar circuit. However, a recent report demonstrated a ET patient with pathologically-confirmed inferior olive degeneration [107], raising the question whether the inferior olive is required for tremor. On the other hand, a quantitative postmortem analysis on the neuronal number in the inferior olive did not show any neurodegeneration in ET [108], suggesting that majority of ET patients may still have functional inferior olivary neurons. The evidence suggests heterogeneity in ET, and not all ET patients have hyper-active inferior olivary neurons. Further studies on gap junctions, glutamatergic and GABAergic components in the inferior olive of the postmortem ET brain will provide further insight.
6.2. Cerebellar GABA deficiency
The link between GABA-mediated mechanism and tremor is supported by the therapeutic effect of GABAergic medications and alcohol in tremor [34], and approximately two thirds of ET patients report tremor responsiveness to alcohol intake [86]. Therefore, insufficient GABA neurotransmission has been postulated in ET. Where is the location of GABAergic dysfunction in the ET brain? Several locations have been postulated within the cerebellar circuit: 1) deep cerebellar nuclei, 2) cerebellar cortex, 3) inferior olive, and we will discuss each evidence as below.
One of the pathological features of ET is modest Purkinje cell loss [109]. Since Purkinje cells are GABAergic, this can lead to insufficient GABAergic neurotransmission to the deep cerebellar nuclei. However, no detailed pathological analysis of Purkinje cell terminals or synaptic numbers onto the deep cerebellar nuclei levels in the postmortem ET brain has been performed. On the other hand, a magnetic resonance spectroscopy (MRS) study did not show differences of GABA concentration in the dentate nuclei between ET patients and controls [110]. A postmortem analysis of ET dentate nuclei demonstrated reduced levels of GABAA and GABAB receptors [84], which probably predicts less responsiveness to GABAergic agents. Therefore, more complex GABAergic mechanism has been proposed that a compensatory up- regulation of extra-synaptic GABA receptors may occur, explaining alcohol responsiveness. A computational modeling study, simulating the effects of progressive loss of GABAA α1 receptor subunits and a compensatory up-regulation of GABAA α2/3 receptor subunits, suggest such changes can produce neuronal oscillatory activity and subsequently tremor [111]. However, the compensatory up-regulation of specific types of GABA receptors have not been demonstrated in the deep cerebellar nuclei in the postmortem ET brain, which will be an important topic for future studies.
The second location for potential GABAergic dysregulation is in the cerebellar cortex (i.e., Purkinje cells). Purkinje cells have strong pacemaking activity, which is tightly regulated by GABAergic neurotransmission from various interneurons, including stellate cells and basket cells [112]. Loss of GABAergic inputs from these cells can drastically alter Purkinje cell firing patterns. In the postmortem examination, basket cells in the ET cerebellum form dense and complex axonal processes surrounding Purkinje cell axonal initial segment [76], which is likely to alter Purkinje cell physiology. Another independent evidence comes from a genome-wide association study (GWAS), which identified a gene called leucine rich repat and immunoglobulin-like domain-containing protein 1 (LINGO1) to be associated with ET [113]. While LINGO-1 is important to regulate myelination [114], LINGO-1 is also enriched in the basket cell axonal processes. Interestingly, LINGO-1 expressing basket cell processes appear to be particularly elongated in the ET cerebellum [75], providing another clues for a roles of cerebellar interneurons in ET. Purkinje cell physiology is likely to be regulated by these interneurons via GABA receptors. Interestingly, knocking out GABAA receptor α1 subunit in mice can lead to tremor [115], and a follow-up study showed that Purkinje cell-specific GABAA receptor α1 subunit knockout is sufficient to produce such tremor [116], further supporting the importance of GABAergic neurotransmission onto Purkinje cells in tremor. However, we still do not have a clear understanding of the exact site of action (dendritic vs. soma vs. axons) of these GABAergic receptors on Purkinje cells, and the specific GABA receptor subtypes involved remain undetermined. While basket cell axonal processes express glutamate decarboxylase [75], suggesting GABAergic in nature, it is unclear whether the axonal initial segment of Purkinje cell expresses GABA receptors or not. It has been suggested that basket cells regulate Purkinje cell axonal initial segments via ephaptic mechanism by altering the electric field, rather than via GABAergic neurotransmission [117]. If this is the case, tremor in Purkinje cell-specific GABAA receptor α1 subunit knockout mice [116] is unlikely to come from basket cell-Purkinje cell axonal initial segment interface.
The third possible location is the inferior olive, which receives GABAergic inputs from the deep cerebellar nuclei. These GABAergic inputs can regulate the synchrony of neuronal firing of inferior olive, which in turn affect the complex spike timing and frequency in the downstream Purkinje cells [100]. Loss of such GABAergic inputs has been proposed to cause inferior olivary hypertrophy and oculopalatal tremor [118,119]. This part of GABAergic dysfunction is less studied in ET.
To further determine the location of GABAergic dysfunction in ET, a PET study using 11C-flumazenil to assess GABAergic neurotransmission showed increased ligand binding in the ET cerebellum, ventrolateral thalamus, and the premotor cortex, suggesting the dysregulation of GABA in multiple areas outside of the cerebellum can also be important in tremor [120]. Currently, a positive allosteric modulator of synaptic and extra-synaptic GABAA receptors is being tested in a clinical trial [121], which can be a novel therapeutic agent for ET.
6.3. GluRδ2-dependent climbing fiber synaptic pathology and cerebellar oscillations
While several neuropathological features of ET have been reported, the most distinguishing pathological feature is the extension of climbing fibers into the parallel fiber synaptic territory (Fig. 1), which is not seen in cerebellar ataxic disorders such as spinocerebellar ataxia or multiple system atrophy [79]. Climbing fiber and parallel fiber innervating territory on Purkinje cell dendrites is strictly controlled by a master synaptic organizer, called GluRδ2. GluRδ2 deficiency in mice can cause abnormal expansion of climbing fiber synaptic territory [122]. Consistently, ET cerebellum has reduced GluRδ2 levels, and the reduction of GluRδ2 protein levels correlates with the climbing fiber synaptic pathology, suggesting that ET patients may have GluRδ2-mediated climbing fiber synaptic pathology [41]. To simulate the effects of GluRδ2 deficiency and climbing fiber synaptic pathology in tremor, a natural mutant mouse, Grid2dupE3 mice was studied, which also have ET- like GluRδ2 deficiency and climbing fiber synapses extending into the parallel fiber synaptic territory in the cerebellum. Interestingly, Grid2dupE3 mice developed adult onset, progressive, and action-dependent tremor that is responsive to primidone, propranolol and alcohol [41], establishing convincing evidence that GluRδ2-dependent climbing fiber synaptic pathology can generate ET-like tremor.
Using in vivo recording technique, Grid2dupE3 mouse cerebellum was found to have oscillatory activity, coherent with tremor. To answer whether this oscillatory activity in the cerebellum is sufficient to generate tremor, an experiment using optogenetic technique to re-create such cerebellar oscillatory activity has been performed in the wild type mice without any climbing fiber synaptic pathology. Interestingly, optogenetically-driven cerebellar oscillatory activity in wild type mice is sufficient to create tremor, demonstrating the causative relationship between abnormal cerebellar physiology and tremor [41]. Since cerebellar oscillatory activity appears to be an important physiological signature for ET, a new technique, cerebellar EEG, has been developed to detect cerebellar oscillatory activity. Consistently, cerebellar EEG can record oscillatory activity in ET patients, and the strength of cerebellar oscillatory activity correlates with tremor severity [41]. A follow up study further demonstrated that cerebellar oscillatory activity exists in both familial as well as sporadic ET patients [123], suggesting this physiological feature can be found in different ET population.
Another related evidence of excitatory synaptic transmission involvement in ET comes from genetic studies. A GWAS identified the association of ET with SLC1A2 [124], which encodes excitatory amino acid transporter type 2 (EAAT2). EAAT2 is expressed by astrocytes to uptake excessive glutamate from excitatory neuronal transmission to prevent over-excitation. While EAAT1 is a predominant type of EAAT in the cerebellar astrocytes, EAAT2 is also expressed [125]. In the cerebellum, climbing fiber-Purkinje cell synapses are one of the most powerful excitatory synaptic inputs in the central nervous system, and the activation of such synapses elicits complex spikes in Purkinje cells with strong calcium influx. Therefore, EAATs play an important role to protect Purkinje cells from long-term over-excitation. Interestingly, ET cerebellar cortex has reduced EAAT2 expression [125,126], along with hyperinnervation of climbing fiber-Purkinje cell synapses [77]. Thus, these alterations may render Purkinje cells particularly vulnerable, leading to Purkinje cell damages and contributing to disease progression.
There are still several unanswered questions of GluRδ2-mediated climbing fiber synaptic pathology in ET. Genetic mutations of Grid2 gene, which lead to a complete disruption of GluRδ2 protein, cause autosomal recessive spinocerebellar ataxia type 18 (SCAR18) in human (OMIM 616204) with prominent ataxia and occasional tremor [127]. However, ET patients have very different clinical presentations, with prominent tremor and occasionally subtle signs of ataxia such as difficulty in tandem gait [128]. The distinctive clinical features of ataxia and tremor spectrum may be attributed to the developmental process because ET is predominantly an adult onset disorder as SCAR18 is a childhood onset disease. It is possible that GluRδ2 is critical in the developmental stage, and complete loss of this protein congenitally could lead to severe cerebellar dysfunction. Another open question is why ET patients have GluRδ2 deficiency? This can possibly due to a complex genetic-environmental interaction in the post-developmental stage. Of note, auto-antibody of GluRδ2 is associated a rare form of encephalitis, which can present as tremor and opsoclonus [129], supporting the post-developmental modulation in tremor pathophysiology.
6.4. Extra-cerebellar oscillatory activity
The above-mentioned theories focus on the single oscillatory source from the cerebellum in ET. However, there are other alternative theories that oscillators can exist outside of the cerebellum, and/or multiple oscillators may interact with each other to create tremor. These theories are supported by the following observational studies in ET patients. First, single neuron recordings in the thalamus of ET patients have been shown to be highly correlated with tremor [130], suggesting that thalamus may serve as the oscillatory activity generator. Within the thalamus, local field potential recordings showed distinct “tremor clusters,” suggesting that multiple oscillators may cooperate to create tremor, outside of the cerebellum [131]. Using EEG-EMG coherent source analysis in ET patients, bi-directional thalamo-cortical oscillations are proposed as the mechanism for tremor [132], indicating that multiple oscillators may also interact in the long-ranged thalamo-cortical loop. Finally, an interesting case report described a 9-year old girl who developed unilateral postural and kinetic tremor after ipsilateral near-total cerebellar hemispherectomy [133]. This suggests that oscillators can be independent of the cerebellum. Moreover, tremor in this case did not develop immediately after cerebellectomy. Rather, there was a 7-year delay between cerebellectomy and tremor development, suggesting a re-organization of the brain circuitry [133].
While thalamus and/or motor cortex may be postulated to be oscillators for tremor generation, to our knowledge, there is no convincing animal model evidence to demonstrate the causal relationship that oscillatory activity in these regions can actually drive tremor. In addition, there is no study investigating the pathological changes in the postmortem thalamus and/or motor cortex in ET patients to further provide structural basis of oscillatory activity in these brain regions.
7. Conclusion
Recent advances in our understanding of ET have revealed multiple converging lines of evidence of heterogenous clinical features and possibly distinctive pathophysiology. As we gradually gain a clearer picture of cerebellar dysfunction in ET, it is likely that specific therapeutic development will be geared towards a segment of ET population with defined pathophysiology. Therefore, developing physiological tools and/or biomarker measurements will be an important next step to probe ET heterogeneity and to prime for successful clinical trials.
Funding source
Dr. Pan has received funding from the Ministry of Science and Technology in Taiwan, grants MOST 109-2326-B-002-013-MY4 (principal investigator), MOST 107-2321-B-002-020 (principal investigator), MOST 108-2321-B-002-011 (principal investigator), and MOST 108-2321-002-059-MY2 (principal investigator). Dr. Kuo has received funding from the National Institutes of Health: NINDS #R01 NS104423 (principal investigator), #R01 NS118179 (principal investigator), NINDS #R03 NS114871 (principal investigator), National Ataxia Foundation, and International Essential Tremor Foundation.
Footnotes
Declaration of Competing Interest
Dr. Pan & Dr. Kuo serve as scientific advisor for Praxis Precision Medicines. Dr. Kuo serves as scientific advisor for Sage Therapeutics for tremor therapy development.
References
- [1].Song P, et al. , The global prevalence of essential tremor, with emphasis on age and sex: a meta-analysis, J. Glob. Health 11 (2021) 04028, 10.7189/jogh.11.04028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Louis ED, McCreary M, How common is essential tremor? Update on the worldwide prevalence of essential tremor, Tremor. Other Hyperkinet Mov. (N. Y.) 11 (2021) 28, 10.5334/tohm.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Louis ED, Dogu O, Does age of onset in essential tremor have a bimodal distribution? Data from a tertiary referral setting and a population-based study, Neuroepidemiology 29 (2007) 208–212, 10.1159/000111584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hopfner F, et al. , Early- and late-onset essential tremor patients represent clinically distinct subgroups, Mov. Disord 31 (2016) 1560–1566, 10.1002/mds.26708. [DOI] [PubMed] [Google Scholar]
- [5].Louis ED, et al. , Older onset essential tremor: more rapid progression and more degenerative pathology, Mov. Disord 24 (2009) 1606–1612, 10.1002/mds.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Muthuraman M, et al. , Essential and aging-related tremor: differences of central control, Mov. Disord 30 (2015) 1673–1680, 10.1002/mds.26410. [DOI] [PubMed] [Google Scholar]
- [7].Kuo SH, et al. , Cerebellar pathology in early onset and late onset essential tremor, Cerebellum 16 (2017) 473–482, 10.1007/s12311-016-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Bhatia KP, et al. , Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society, Mov. Disord 33 (2018) 75–87, 10.1002/mds.27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Rajalingam R, Breen DP, Lang AE, Fasano A, Essential tremor plus is more common than essential tremor: insights from the reclassification of a cohort of patients with lower limb tremor, Parkinsonism Relat. Disord 56 (2018) 109–110, 10.1016/j.parkreldis.2018.06.029. [DOI] [PubMed] [Google Scholar]
- [10].Prasad S, Pal PK, Reclassifying essential tremor: implications for the future of past research, Mov. Disord 34 (2019) 437, 10.1002/mds.27615. [DOI] [PubMed] [Google Scholar]
- [11].Louis ED, et al. , Essential tremor-plus: a controversial new concept, Lancet Neurol. 19 (2020) 266–270, 10.1016/s1474-4422(19)30398-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Louis ED, “essential tremor plus”: a problematic concept: implications for clinical and epidemiological studies of essential tremor, Neuroepidemiology 54 (2020) 180–184, 10.1159/000502862. [DOI] [PubMed] [Google Scholar]
- [13].Elble RJ, Essential tremor is a useful concept? No. Mov. Disord. Clin. Pract 4 (2017) 663–665, 10.1002/mdc3.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gionco JT, et al. , Essential tremor versus “ET-plus”: a detailed postmortem study of cerebellar pathology, Cerebellum (2021), 10.1007/s12311-021-01263-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Louis ED, Huey ED, Cosentino S, Features of “ET plus” correlate with age and tremor duration: “ET plus” may be a disease stage rather than a subtype of essential tremor, Parkinsonism Relat. Disord 91 (2021) 42–47, 10.1016/j.parkreldis.2021.08.017. [DOI] [PubMed] [Google Scholar]
- [16].Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ, Essential tremor: predictors of disease progression in a clinical cohort, J. Neurol. Neurosurg. Psychiatry 77 (2006) 1235–1237, 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zesiewicz TA, Kuo SH, Essential tremor, BMJ Clin. Evid 2015 (2015). [PMC free article] [PubMed] [Google Scholar]
- [18].Hedera P, Cibulčík F, Davis TL, Pharmacotherapy of essential tremor, J. Cent. Nerv. Syst. Dis 5 (2013) 43–55, 10.4137/jcnsd.S6561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ondo WG, et al. , Topiramate in essential tremor: a double-blind, placebo- controlled trial, Neurology 66 (2006) 672–677, 10.1212/01.wnl.0000200779.03748.0f. [DOI] [PubMed] [Google Scholar]
- [20].Jog M, et al. , Tolerability and efficacy of customized IncobotulinumtoxinA injections for essential tremor: a randomized, double-blind, Placebo-controlled study, Toxins (Basel) 12 (2020), 10.3390/toxins12120807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Schuurman PR, et al. , A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor, N. Engl. J. Med 342 (2000) 461–468, 10.1056/nejm200002173420703. [DOI] [PubMed] [Google Scholar]
- [22].Fasano A, Helmich RC, Tremor habituation to deep brain stimulation: underlying mechanisms and solutions, Mov. Disord 34 (2019) 1761–1773, 10.1002/mds.27821. [DOI] [PubMed] [Google Scholar]
- [23].Favilla CG, et al. , Worsening essential tremor following deep brain stimulation: disease progression versus tolerance, Brain 135 (2012) 1455–1462, 10.1093/brain/aws026. [DOI] [PubMed] [Google Scholar]
- [24].Paschen S, et al. , Long-term efficacy of deep brain stimulation for essential tremor: an observer-blinded study, Neurology 92 (2019) e1378–e1386, 10.1212/wnl.0000000000007134. [DOI] [PubMed] [Google Scholar]
- [25].Elias WJ, et al. , A randomized trial of focused ultrasound Thalamotomy for essential tremor, N. Engl. J. Med 375 (2016) 730–739, 10.1056/NEJMoa1600159. [DOI] [PubMed] [Google Scholar]
- [26].Sinai A, et al. , Magnetic resonance-guided focused ultrasound thalamotomy for essential tremor: a 5-year single-center experience, J. Neurosurg 1–8 (2019), 10.3171/2019.3.Jns19466. [DOI] [PubMed] [Google Scholar]
- [27].Kuo SH, et al. , Deep brain stimulation and climbing fiber synaptic pathology in essential tremor, Ann. Neurol 80 (2016) 461–465, 10.1002/ana.24728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cao H, Thompson-Westra J, Hallett M, Haubenberger D, The response of the central and peripheral tremor component to octanoic acid in patients with essential tremor, Clin. Neurophysiol 129 (2018) 1467–1471, 10.1016/j.clinph.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Elble RJ, Characteristics of physiologic tremor in young and elderly adults, Clin. Neurophysiol 114 (2003) 624–635, 10.1016/s1388-2457(03)00006-3. [DOI] [PubMed] [Google Scholar]
- [30].Vial F, Kassavetis P, Merchant S, Haubenberger D, Hallett M, How to do an electrophysiological study of tremor, Clin. Neurophysiol. Pract 4 (2019) 134–142, 10.1016/j.cnp.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Elble RJ, Higgins C, Leffler K, Hughes L, Factors influencing the amplitude and frequency of essential tremor, Mov. Disord 9 (1994) 589–596, 10.1002/mds.870090602. [DOI] [PubMed] [Google Scholar]
- [32].van der Veen S, et al. , The diagnostic value of clinical neurophysiology in hyperkinetic movement disorders: a systematic review, Parkinsonism Relat. Disord 89 (2021) 176–185, 10.1016/j.parkreldis.2021.07.033. [DOI] [PubMed] [Google Scholar]
- [33].Zhang J, Xing Y, Ma X, Feng L, Differential diagnosis of Parkinson disease, essential tremor, and enhanced physiological tremor with the tremor analysis of EMG, Parkinson’s Disease 2017 (2017) 1597907, 10.1155/2017/1597907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Haubenberger D, Hallett M, Essential Tremor N. Engl. J. Med 378 (2018) 1802–1810, 10.1056/NEJMcp1707928. [DOI] [PubMed] [Google Scholar]
- [35].Pan MK, Kuo SH, Tracking the central and peripheral origin of tremor, Clin. Neurophysiol 129 (2018) 1451–1452, 10.1016/j.clinph.2018.04.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Panyakaew P, Cho HJ, Lee SW, Wu T, Hallett M, The pathophysiology of dystonic tremors and comparison with essential tremor, J. Neurosci 40 (2020) 9317–9326, 10.1523/jneurosci.1181-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dirkx MF, Zach H, Bloem BR, Hallett M, Helmich RC, The nature of postural tremor in Parkinson disease, Neurology 90 (2018) e1095–e1103, 10.1212/wnl.0000000000005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].di Biase L, et al. , Tremor stability index: a new tool for differential diagnosis in tremor syndromes, Brain J. Neurol 140 (2017) 1977–1986, 10.1093/brain/awx104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Schnitzler A, Münks C, Butz M, Timmermann L, Gross J, Synchronized brain network associated with essential tremor as revealed by magnetoencephalography, Mov. Disord 24 (2009) 1629–1635, 10.1002/mds.22633. [DOI] [PubMed] [Google Scholar]
- [40].Saranza G, Fasano A, Excessive cerebellar oscillations in essential tremor: insights into disease mechanism and treatment, Mov. Disord 35 (2020) 758, 10.1002/mds.28040. [DOI] [PubMed] [Google Scholar]
- [41].Pan MK, et al. , Cerebellar oscillations driven by synaptic pruning deficits of cerebellar climbing fibers contribute to tremor pathophysiology, Sci. Transl. Med 12 (2020), 10.1126/scitranslmed.aay1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Connolly AT, Bajwa JA, Johnson MD, Cortical magnetoencephalography of deep brain stimulation for the treatment of postural tremor, Brain Stimul. 5 (2012) 616–624, 10.1016/j.brs.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Buijink AW, et al. , Motor network disruption in essential tremor: a functional and effective connectivity study, Brain J. Neurol 138 (2015) 2934–2947, 10.1093/brain/awv225. [DOI] [PubMed] [Google Scholar]
- [44].Le Pira F, Giuffrida S, Panetta MR, Lo Bartolo ML, Politi G, Selective disappearance of essential tremor after ischaemic stroke, Eur. J. Neurol 11 (2004) 422–423, 10.1111/j.1468-1331.2004.00824.x. [DOI] [PubMed] [Google Scholar]
- [45].Dupuis MJ, Delwaide PJ, Boucquey D, Gonsette RE, Homolateral disappearance of essential tremor after cerebellar stroke, Mov. Disord 4 (1989) 183–187, 10.1002/mds.870040210. [DOI] [PubMed] [Google Scholar]
- [46].Dupuis MJ, Evrard FL, Jacquerye PG, Picard GR, Lermen OG, Disappearance of essential tremor after stroke, Mov. Disord 25 (2010) 2884–2887, 10.1002/mds.23328. [DOI] [PubMed] [Google Scholar]
- [47].Hellwig B, et al. , Tremor-correlated cortical activity in essential tremor, Lancet 357 (2001) 519–523. [DOI] [PubMed] [Google Scholar]
- [48].Sharifi S, et al. , Directionality of corticomuscular coupling in essential tremor and cortical myoclonic tremor, Clin. Neurophysiol 132 (2021) 1878–1886, 10.1016/j.clinph.2021.04.011. [DOI] [PubMed] [Google Scholar]
- [49].Muthuraman M, et al. , Cerebello-cortical network fingerprints differ between essential, Parkinson’s and mimicked tremors, Brain 141 (2018) 1770–1781, 10.1093/brain/awy098. [DOI] [PubMed] [Google Scholar]
- [50].Bucher SF, Seelos KC, Dodel RC, Reiser M, Oertel WH, Activation mapping in essential tremor with functional magnetic resonance imaging, Ann. Neurol 41 (1997) 32–40, 10.1002/ana.410410108. [DOI] [PubMed] [Google Scholar]
- [51].Neely KA, et al. , Functional brain activity relates to 0–3 and 3–8 Hz force oscillations in essential tremor, Cereb. Cortex 25 (2015) 4191–4202, 10.1093/cercor/bhu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Broersma M, et al. , Bilateral cerebellar activation in unilaterally challenged essential tremor, NeuroImage. Clin 11 (2016) 1–9, 10.1016/j.nicl.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Buijink AW, et al. , Rhythmic finger tapping reveals cerebellar dysfunction in essential tremor, Parkinsonism Relat. Disord 21 (2015) 383–388, 10.1016/j.parkreldis.2015.02.003. [DOI] [PubMed] [Google Scholar]
- [54].Holtbernd F, Shah NJ, Imaging the pathophysiology of essential tremor-a systematic review, Front. Neurol 12 (2021), 680254, 10.3389/fneur.2021.680254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Yin W, Lin W, Li W, Qian S, Mou X, Resting state fMRI demonstrates a disturbance of the Cerebello-cortical circuit in essential tremor, Brain Topogr. 29 (2016) 412–418, 10.1007/s10548-016-0474-6. [DOI] [PubMed] [Google Scholar]
- [56].Benito-Leon J, et al. , Graph theory analysis of resting-state functional magnetic ´ resonance imaging in essential tremor, Hum. Brain Mapp 40 (2019) 4686–4702, 10.1002/hbm.24730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Fang W, et al. , Essential tremor is associated with disruption of functional connectivity in the ventral intermediate Nucleus–Motor Cortex–Cerebellum circuit, Hum. Brain Mapp 37 (2016) 165–178, 10.1002/hbm.23024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Tikoo S, et al. , Functional disconnection of the dentate nucleus in essential tremor, J. Neurol 267 (2020) 1358–1367, 10.1007/s00415-020-09711-9. [DOI] [PubMed] [Google Scholar]
- [59].Mueller K, et al. , General and selective brain connectivity alterations in essential tremor: a resting state fMRI study, NeuroImage. Clin 16 (2017) 468–476, 10.1016/j.nicl.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Lenka A, et al. , Role of altered cerebello-thalamo-cortical network in the neurobiology of essential tremor, Neuroradiology 59 (2017) 157–168, 10.1007/s00234-016-1771-1. [DOI] [PubMed] [Google Scholar]
- [61].Nicoletti V, et al. , Cerebello-thalamo-cortical network is intrinsically altered in essential tremor: evidence from a resting state functional MRI study, Sci. Rep 10 (2020) 16661, 10.1038/s41598-020-73714-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Wang L, et al. , Resting-state fMRI study on drug-naive patients of essential tremor with and without head tremor, Sci. Rep 8 (2018) 10580, 10.1038/s41598-018-28778-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lu MK, et al. , Resetting tremor by single and paired transcranial magnetic stimulation in Parkinson’s disease and essential tremor, Clin. Neurophysiol 126 (2015) 2330–2336, 10.1016/j.clinph.2015.02.010. [DOI] [PubMed] [Google Scholar]
- [64].Yu HY, et al. , Single-pulse transcranial magnetic stimulation reset the rhythm of essential tremor but not heart beat. Zhonghua yi xue za zhi =, Chin. Med. J. Free China Ed 64 (2001) 271–276. [PubMed] [Google Scholar]
- [65].Schreglmann SR, et al. , Non-invasive suppression of essential tremor via phase- locked disruption of its temporal coherence, Nat. Commun 12 (2021) 363, 10.1038/s41467-020-20581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Cagnan H, et al. , Phase dependent modulation of tremor amplitude in essential tremor through thalamic stimulation, Brain 136 (2013) 3062–3075, 10.1093/brain/awt239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Cagnan H, et al. , The nature of tremor circuits in parkinsonian and essential tremor, Brain 137 (2014) 3223–3234, 10.1093/brain/awu250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cagnan H, et al. , Stimulating at the right time: phase-specific deep brain stimulation, Brain 140 (2017) 132–145, 10.1093/brain/aww286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Louis EDE, et al. , Neuropathological changes in essential tremor: 33 cases compared with 21 controls, Brain 130 (2007) 3297–3307, 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- [70].Kuo SH, et al. , Increased number of heterotopic Purkinje cells in essential tremor, J. Neurol. Neurosurg. Psychiatry 82 (2011) 1038–1040, 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Babij R, et al. , Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains, Brain 136 (2013) 3051–3061, 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Yu M, et al. , Increased number of Purkinje cell dendritic swellings in essential tremor, Eur. J. Neurol 19 (2012) 625–630, 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Louis ED, et al. , Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor, Brain 137 (2014) 3142–3148, 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Lang-Ouellette D, et al. , Purkinje cell axonal swellings enhance action potential fidelity and cerebellar function, Nat. Commun 12 (2021) 4129, 10.1038/s41467-021-24390-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kuo S-H, et al. , Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau, Acta Neuropathol. 125 (2013) 879–889, 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Erickson-Davis CRC, et al. , Hairy baskets associated with degenerative Purkinje cell changes in essential tremor, J. Neuropathol. Exp. Neurol 69 (2010) 262–271, 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Lin C-Y, et al. , Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum, Brain 137 (2014) 3149–3159, 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Louis RJ, Lin C-Y, Faust PL, Koeppen AH, Kuo S-H, Climbing Fiber Synaptic Changes Correlate with Clinical Features in Essential Tremor 84, 2015, 10.1212/WNL.0000000000001636-0000000000002286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kuo SH, et al. , Climbing fiber-Purkinje cell synaptic pathology in tremor and cerebellar degenerative diseases, Acta Neuropathol. 133 (2017) 121–138, 10.1007/s00401-016-1626-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Louis ED, et al. , Contextualizing the pathology in the essential tremor cerebellar cortex: a patholog-omics approach, Acta Neuropathol. 138 (2019) 859–876, 10.1007/s00401-019-02043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Lee D, Gan SR, Faust PL, Louis ED, Kuo SH, Climbing fiber-Purkinje cell synaptic pathology across essential tremor subtypes, Parkinsonism Relat. Disord (2018), 10.1016/j.parkreldis.2018.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Wu YC, et al. , Increased climbing Fiber lateral crossings on Purkinje cell dendrites in the cerebellar hemisphere in essential tremor, Mov. Disord 36 (2021) 1440–1445, 10.1002/mds.28502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hartstone WG, et al. , Dentate nucleus neuronal density: a postmortem study of essential tremor versus control brains, Mov. Disord 36 (2021) 995–999, 10.1002/mds.28402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Paris-Robidas S, et al. , Defective dentate nucleus GABA receptors in essential tremor, Brain 135 (2012) 105–116, 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- [85].Martuscello RT, et al. , Gene expression analysis of the cerebellar cortex in essential tremor, Neurosci. Lett 721 (2020), 134540, 10.1016/j.neulet.2019.134540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lou JS, Jankovic J, Essential tremor: clinical correlates in 350 patients, Neurology 41 (1991) 234–238. [DOI] [PubMed] [Google Scholar]
- [87].Ong YL, Deng X, Tan EK, Etiologic links between environmental and lifestyle factors and essential tremor, Ann. Clin. Transl. Neurol 6 (2019) 979–989, 10.1002/acn3.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Louis ED, et al. , Elevated blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentrations in essential tremor, Neurotoxicology 29 (2008) 294–300, 10.1016/j.neuro.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Louis ED, Zheng W, Applegate L, Shi L, Factor-Litvak P, Blood harmane concentrations and dietary protein consumption in essential tremor, Neurology 65 (2005) 391–396, 10.1212/01.wnl.0000172352.88359.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Louis ED, et al. , Blood harmane (1-methyl-9H-pyrido[3,4-b]indole) concentration in essential tremor cases in Spain, Neurotoxicology 34 (2013) 264–268, 10.1016/j.neuro.2012.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Louis ED, et al. , Elevated brain harmane (1-methyl-9H-pyrido[3,4-b]indole) in essential tremor cases vs. controls, Neurotoxicology 38C (2013) 131–135, 10.1016/j.neuro.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pan MK, Ni CL, Wu YC, Li YS, Kuo SH, Animal models of tremor: relevance to human tremor disorders, Tremor. Other Hyperkinet Mov. (N. Y.) 8 (2018) 587, 10.7916/d89s37mv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Paterson NE, Malekiani SA, Foreman MM, Olivier B, Hanania T, Pharmacological characterization of harmaline-induced tremor activity in mice, Eur. J. Pharmacol 616 (2009) 73–80, 10.1016/j.ejphar.2009.05.031. [DOI] [PubMed] [Google Scholar]
- [94].Du W, Aloyo VJ, Harvey JA, Harmaline competitively inhibits [3H]MK-801 binding to the NMDA receptor in rabbit brain, Brain Res. 770 (1997) 26–29. [DOI] [PubMed] [Google Scholar]
- [95].Handforth A, Harmaline tremor: underlying mechanisms in a potential animal model of essential tremor, Tremor. Other Hyperkinet Mov. (N. Y.) 2 (2012), 10.7916/d8td9w2p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Martin FC, Handforth A, Carbenoxolone and mefloquine suppress tremor in the harmaline mouse model of essential tremor, Mov. Disord 21 (2006) 1641–1649, 10.1002/mds.20940. [DOI] [PubMed] [Google Scholar]
- [97].De Gruijl JR, Sokol PA, Negrello M, De Zeeuw CI, Modulation of electrotonic coupling in the inferior olive by inhibitory and excitatory inputs: integration in the glomerulus, Neuron 81 (2014) 1215–1217, 10.1016/j.neuron.2014.03.009. [DOI] [PubMed] [Google Scholar]
- [98].Lefler Y, Yarom Y, Uusisaari MY, Cerebellar inhibitory input to the inferior olive decreases electrical coupling and blocks subthreshold oscillations, Neuron 81 (2014) 1389–1400, 10.1016/j.neuron.2014.02.032. [DOI] [PubMed] [Google Scholar]
- [99].Marshall SP, Lang EJ, Local changes in the excitability of the cerebellar cortex produce spatially restricted changes in complex spike synchrony, J. Neurosci 29 (2009) 14352–14362, 10.1523/jneurosci.3498-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Lang EJ, GABAergic and glutamatergic modulation of spontaneous and motor- cortex-evoked complex spike activity, J. Neurophysiol 87 (2002) 1993–2008, 10.1152/jn.00477.2001. [DOI] [PubMed] [Google Scholar]
- [101].Park YG, et al. , Ca(V)3.1 is a tremor rhythm pacemaker in the inferior olive, Proc. Natl. Acad. Sci. U. S. A 107 (2010) 10731–10736, 10.1073/pnas.1002995107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Odgerel Z, et al. , Whole genome sequencing and rare variant analysis in essential tremor families, PLoS One 14 (2019), e0220512, 10.1371/journal.pone.0220512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Matsumoto-Makidono Y, et al. , Ionic basis for membrane potential resonance in neurons of the inferior olive, Cell Rep. 16 (2016) 994–1004, 10.1016/j.celrep.2016.06.053. [DOI] [PubMed] [Google Scholar]
- [104].Ly R, et al. , T-type channel blockade impairs long-term potentiation at the parallel fiber-Purkinje cell synapse and cerebellar learning, Proc. Natl. Acad. Sci. U. S. A 110 (2013) 20302–20307, 10.1073/pnas.1311686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Handforth A, et al. , T-type calcium channel antagonists suppress tremor in two mouse models of essential tremor, Neuropharmacology 59 (2010) 380–387, 10.1016/j.neuropharm.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Papapetropoulos S, et al. , A phase 2 proof-of-concept, randomized, placebo- controlled trial of CX-8998 in essential tremor, Mov. Disord 36 (2021) 1944–1949, 10.1002/mds.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Louis ED, et al. , Inferior Olivary nucleus degeneration does not lessen tremor in essential tremor, Cerebell. Atax. 5 (2018) 1, 10.1186/s40673-018-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Louis ED, Babij R, Cortes E, Vonsattel J-PG, Faust PL, The inferior olivary nucleus: a postmortem study of essential tremor cases versus controls, Mov. Disord 28 (2013) 779–786, 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Louis ED, Faust PL, Essential tremor pathology: neurodegeneration and reorganization of neuronal connections, Nat. Rev. Neurol 16 (2020) 69–83, 10.1038/s41582-019-0302-1. [DOI] [PubMed] [Google Scholar]
- [110].Louis ED, Hernandez N, Dyke JP, Ma RE, Dydak U, In vivo dentate nucleus gamma-aminobutyric acid concentration in essential tremor vs. controls, Cerebellum 17 (2018) 165–172, 10.1007/s12311-017-0891-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Zhang X, Santaniello S, Role of cerebellar GABAergic dysfunctions in the origins of essential tremor, Proc. Natl. Acad. Sci. U. S. A 116 (2019) 13592–13601, 10.1073/pnas.1817689116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Brown AM, et al. , Molecular layer interneurons shape the spike activity of cerebellar Purkinje cells, Sci. Rep 9 (2019) 1742, 10.1038/s41598-018-38264-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Stefansson H, et al. , Variant in the sequence of the LINGO1 gene confers risk of essential tremor, Nat. Genet 41 (2009) 277–279, 10.1038/ng.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Mi S, et al. , LINGO-1 negatively regulates myelination by oligodendrocytes, Nat. Neurosci 8 (2005) 745–751, 10.1038/nn1460. [DOI] [PubMed] [Google Scholar]
- [115].Kralic JE, et al. , Genetic essential tremor in gamma-aminobutyric acidA receptor alpha1 subunit knockout mice, J. Clin. Invest 115 (2005) 774–779, 10.1172/jci23625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Nietz A, et al. , Selective loss of the GABA(Aα1) subunit from Purkinje cells is sufficient to induce a tremor phenotype, J. Neurophysiol 124 (2020) 1183–1197, 10.1152/jn.00100.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Blot A, Barbour B, Ultra-rapid axon-axon ephaptic inhibition of cerebellar Purkinje cells by the pinceau, Nat. Neurosci 17 (2014) 289–295, 10.1038/nn.3624. [DOI] [PubMed] [Google Scholar]
- [118].Shaikh AG, et al. , Oculopalatal tremor explained by a model of inferior olivary hypertrophy and cerebellar plasticity, Brain J. Neurol 133 (2010) 923–940, 10.1093/brain/awp323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Tilikete C, Desestret V, Hypertrophic olivary degeneration and palatal or Oculopalatal tremor, Front. Neurol 8 (2017) 302, 10.3389/fneur.2017.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Boecker H, et al. , GABAergic dysfunction in essential tremor: an 11C-flumazenil PET study, J. Nucl. Med 51 (2010) 1030–1035, 10.2967/jnumed.109.074120. [DOI] [PubMed] [Google Scholar]
- [121].Martinez Botella G, et al. , Neuroactive steroids. 2. 3α-Hydroxy-3β-methyl-21-(4- cyano-1H-pyrazol-1′-yl)-19-nor-5β-pregnan-20-one (SAGE-217): a clinical next generation neuroactive steroid positive allosteric modulator of the (γ-aminobutyric acid)(a) receptor, J. Med. Chem 60 (2017) 7810–7819, 10.1021/acs.jmedchem.7b00846. [DOI] [PubMed] [Google Scholar]
- [122].Miyazaki T, et al. , Ablation of glutamate receptor GluRdelta2 in adult Purkinje cells causes multiple innervation of climbing fibers by inducing aberrant invasion to parallel fiber innervation territory, J. Neurosci 30 (2010) 15196–15209, 10.1523/jneurosci.0934-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wong SB, et al. , Cerebellar oscillations in familial and sporadic essential tremor, Cerebellum (2021), 10.1007/s12311-021-01309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Thier S, et al. , Polymorphisms in the glial glutamate transporter SLC1A2 are associated with essential tremor, Neurology 79 (2012) 243–248, 10.1212/WNL.0b013e31825fdeed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Lee M, et al. , Decreased EAAT2 protein expression in the essential tremor cerebellar cortex, Acta Neuropathol. Commun 2 (2014) 157, 10.1186/s40478-014-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Wang J, et al. , Excitatory amino acid transporter expression in the essential tremor dentate nucleus and cerebellar cortex: a postmortem study, Parkinsonism Relat. Disord 32 (2016) 87–93, 10.1016/j.parkreldis.2016.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Hills LB, et al. , Deletions in GRID2 lead to a recessive syndrome of cerebellar ataxia and tonic upgaze in humans, Neurology 81 (2013) 1378–1386, 10.1212/WNL.0b013e3182a841a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Cinar N, Sahin S, Okluoglu Onay T, Karsidag S, Balance in essential tremor during tandem gait: is the first mis-step an important finding? J. Clin. Neurosci 20 (2013) 1433–1437, 10.1016/j.jocn.2013.01.013. [DOI] [PubMed] [Google Scholar]
- [129].Jen JC, Lopez I, Baloh RW, Opsoclonus: clinical and immunological features, J. Neurol. Sci 320 (2012) 61–65, 10.1016/j.jns.2012.06.017. [DOI] [PubMed] [Google Scholar]
- [130].Hua SE, Lenz FA, Zirh TA, Reich SG, Dougherty PM, Thalamic neuronal activity correlated with essential tremor, J. Neurol. Neurosurg. Psychiatry 64 (1998) 273–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Pedrosa DJ, et al. , Essential tremor and tremor in Parkinson’s disease are associated with distinct ‘tremor clusters’ in the ventral thalamus, Exp. Neurol 237 (2012) 435–443, 10.1016/j.expneurol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- [132].Muthuraman M, et al. , Oscillating central motor networks in pathological tremors and voluntary movements. What makes the difference? Neuroimage 60 (2012) 1331–1339, 10.1016/j.neuroimage.2012.01.088. [DOI] [PubMed] [Google Scholar]
- [133].Chahine LM, Ghosh D, Essential tremor after ipsilateral cerebellar hemispherectomy: support for the thalamus as the central oscillator, J. Child Neurol 24 (2009) 861–864, 10.1177/0883073808329528. [DOI] [PubMed] [Google Scholar]