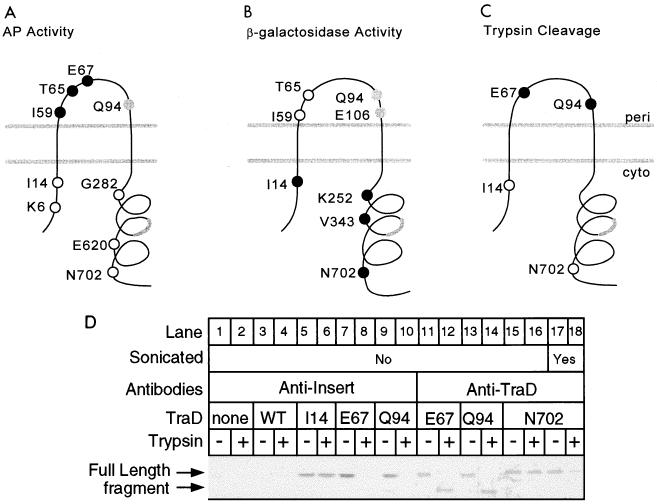
FIG. 2.
Analysis of TraD topology. (A) Locations in the favored two-span model and alkaline phosphatase activities of nine TraD-alkaline phosphatase hybrids. Closed circles denote high activity (greater than 150 Miller units), open circles denote low activity (less than 25 Miller units), and the gray circle denotes an intermediate value (46 Miller units) (Table 2). The thick gray line denotes the location of a potential third membrane-spanning sequence. (B) Locations and β-galactosidase activities of eight TraD–β-galactosidase hybrids. Closed circles denote high activity (I14, 183 U, K252, 133 U; V343, 145 U; N702, 127 U), open circles denote low activity (I59, 2 U; T65, 6 U), and the gray circles denote intermediate activity (Q94, 48 U; E106, 70 U). (C) Positions in the topology and cleavage by trypsin in spheroplasts of four 31-amino-acid insertions in TraD. Closed circles represent insertions that were trypsin sensitive; open circles represent trypsin-resistant insertions. (D) Western blot of TraD insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against either the inserted sequence or wild-type TraD was used as indicated previously (25). A strain deleted for traD was used as a negative control (none). A strain expressing wild-type TraD (WT) served as a negative control in the antiinsert blot. An aliquot of the spheroplast preparation expressing the N702 insertion derivative was sonicated before the addition of trypsin (lanes 17 and 18). Trypsin treatment procedure 1 was used with cells grown at 37°C.