FIGURE 1.
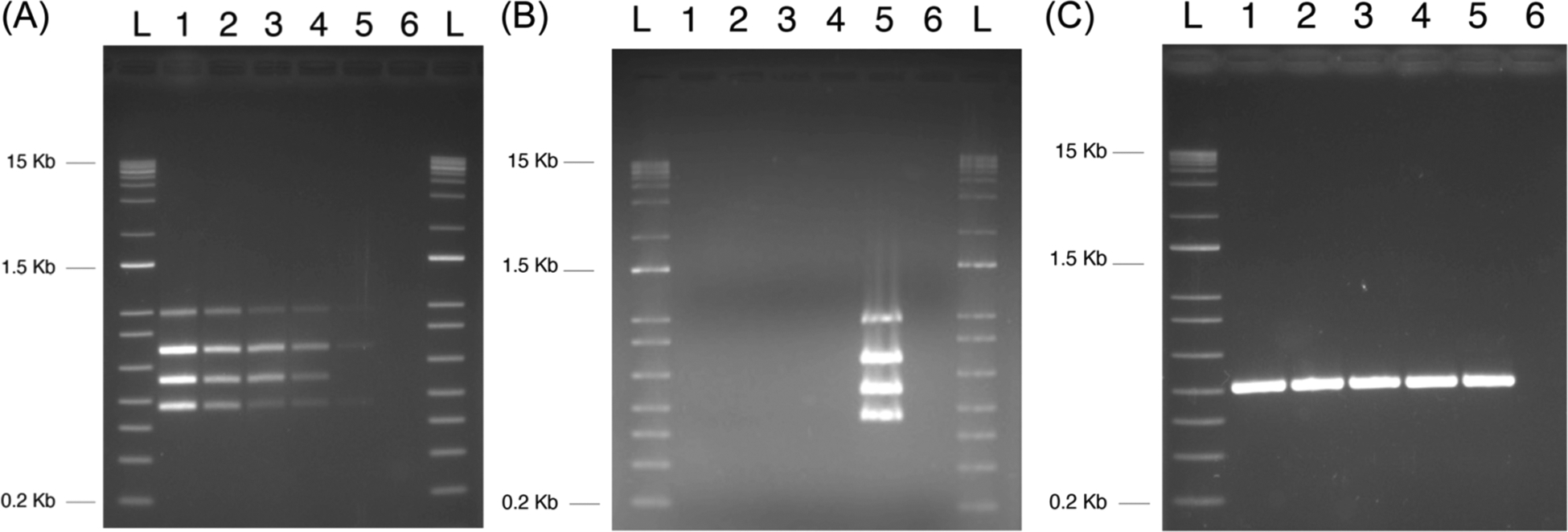
Sensitivity and specificity of the multiplex PCR test for genotoxic bacteria. Multiplex PCR assay development. (A) Assay sensitivity: lane 1 positive control containing 140 CFU/mL, lane 2 positive control containing 70 CFU/mL, lane 3 positive control containing 28 CFU/mL, lane 4 positive control containing 14 CFU/mL, lane 5 positive control containing 2 CFU/mL, and lane 6 no DNA control.
(B) Multiplex assay for marker specificity: lane 1 B1-7-6, lane 2 JJ0055, lane 3 JJ1166, lane 4 JJ1167, lane 5 positive Escherichia coli control for all four markers B1-5-1 and B3-1-4, and lane 6 no DNA control. (C) uidA controls: B1-7-6, lane 2 JJ0055, lane 3 JJ1166, lane 4 JJ1167, lane 5 positive B1-5-1 and B3-1-4, and lane 6 no DNA control. CFU, colony-forming unit; PCR, polymerase chain reaction. L, size ladder.
