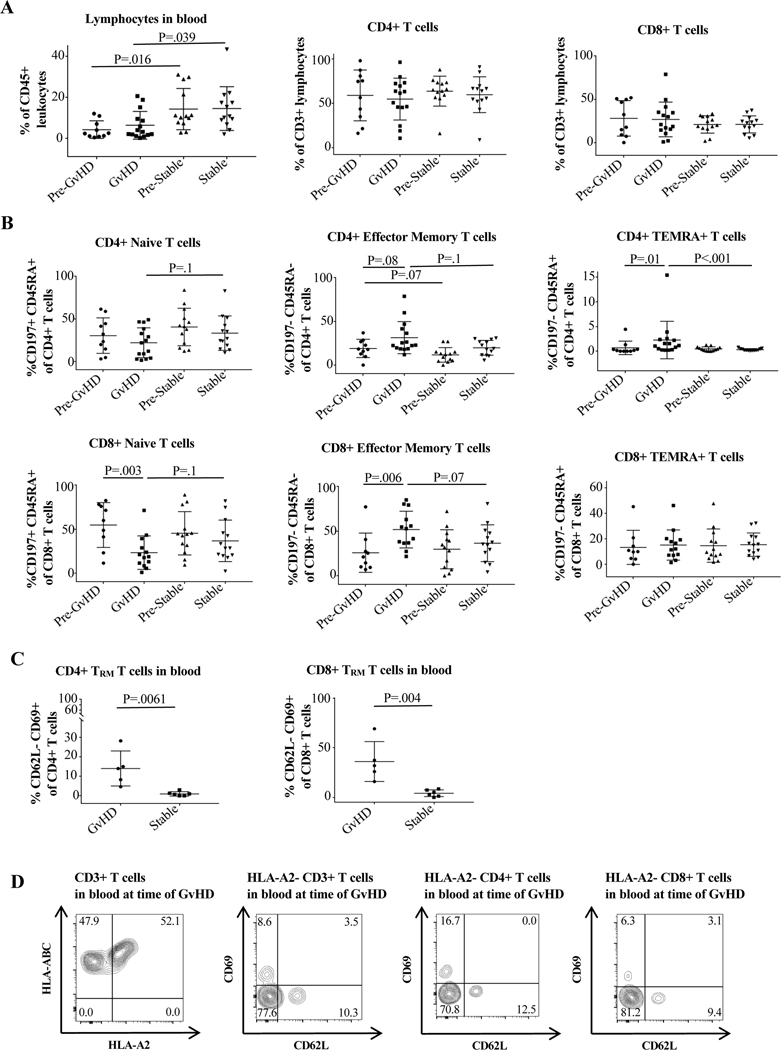
Figure 2. Cell surface marker expression in peripheral blood of GvHD vs. control patients.
Cells were stained and analyzed by flow cytometry as described in Materials and Methods and Supporting Materials and Methods. The GvHD timepoint was defined as the sample taken closest to the time of GvHD clinical manifestation but not after the initiation of treatment. Pre-GvHD was the immediately preceding timepoint. The stable time point was defined as the highest percentage of CD4+ and CD8+ effector memory T cells (TEM) during the post-transplant course in control patients, and the pre-stable timepoint as the timepoint directly preceding this. (A) Differences in overall lymphocyte, CD4+, and CD8+ expression at the different timepoints, with lymphocyte percentage differing significantly between the GvHD and control groups. (B) Differences in CD4+ and CD8+ naïve, TEM, and TEMRA percentages between the groups. CD8+ naïve cell percentage decreased at the time of GvHD, while CD4+TEMRA and CD8+TEM increased. The average percentage of CD4+ and CD8+TEM was large between the groups but only approached statistical significance (p=0.1 for CD4 and p=0.07 for CD8). (C) Statistically significant difference in CD4+ and CD8+CD62L-CD69+TRM in peripheral blood of GvHD compared to control patients. Unpaired/paired t test was used for normally distributed groups; otherwise the non-parametric Mann Whitney/Wilcoxon test was used. For more than two independent groups, the Kruskal-Wallis test was performed followed with Dunn’s multiple comparisons. (D) Flow cytometry plots showing presence of donor derived HLA-A2-CD3+T cells as well as HLA-A2-CD62L-CD69+CD4+ and CD8+TRM in peripheral blood of an HLA-A2+ recipient of an HLA-A2- graft at the time of active GvHD.