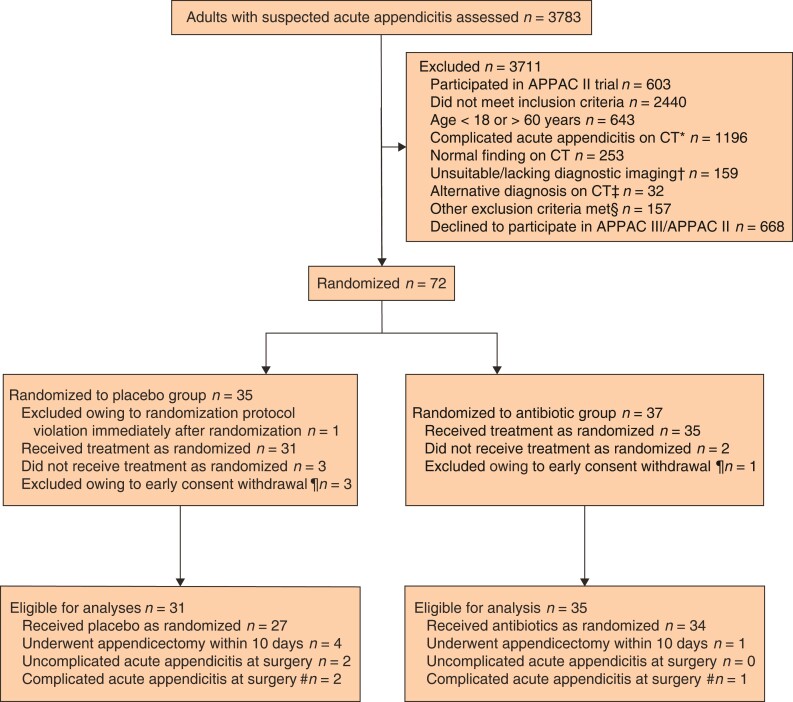
Fig. 1.
Flow chart for APPAC III trial
A total of 71 patients were eligible for the baseline comparison, and 66 for comparison of the primary endpoint. *Includes appendicolith, perforation, abscess, or suspicion of tumour. †Underwent ultrasound imaging, MRI or non-contrast-enhanced CT. ‡Diverticulitis (3 patients), ovarian cyst (7), hernia (2), pelvic inflammatory disease (2), other diagnosis (18). §Pregnancy, lactation, allergy to contrast media, kidney insufficiency, use of metformin, systemic illness, and inability to provide consent. ¶Withdrew consent after randomization but before receiving randomized treatment. #Operative or histopathological findings of appendicolith, gangrene, perforation, abscess, or tumour.