Abstract
Background
Colorectal cancer (CRC) is the second most common solid organ cancer. Traditional treatment is with surgery and chemotherapy. Immunotherapy has recently emerged as a neoadjuvant therapy that could change treatment strategy in both primary resectable and metastatic CRC.
Methods
A literature review of PubMed with a focus on studies exploring upfront immunotherapy in operable CRC, either for primary resectable stage I–III cancers or for (potentially) operable liver metastasis.
Results
Immune checkpoint blockade by the programmed cell death 1 (PD-1) receptor inhibitors nivolumab and pembrolizumab and the cytotoxic T cell-associated protein 4 (CTLA-4) inhibitor ipilimumab has shown good results in both early-stage and advanced CRC. The effects of immune checkpoint inhibitors have so far been demonstrated in small phase I/II studies and predominantly in treatment-refractory stage IV disease with defect Mismatch repair (dMMR). However, recent data from phase I/II (NICHE-1) studies suggest an upfront role for immunotherapy in operable stage I–III disease. By blocking crucial immune checkpoints, cytotoxic T cells are activated and release cytotoxic signals that initiate cancer cell destruction. The very high complete response rate in dMMR operable CRC with neoadjuvant immunotherapy with nivolumab and ipilimumab, and even partial pathological response in some patients with proficient MMR (pMMR) CRC, calls for further attention to patient selection for neoadjuvant treatment, beyond MMR status alone.
Conclusion
Early data on the effect of immunotherapy in CRC provide new strategic thinking of treatment options in CRC for both early-stage and advanced disease, with prospects for new trials.
Immunotherapy has been demonstrated to be effective in subgroups of patients with colorectal cancer. Molecular subtypes may provide an opportunity for new strategic treatment plans, in addition to surgery and chemotherapy.
Introduction
Colorectal cancer (CRC) is the second most common solid organ cancer in both sexes, representing a considerable global health burden. Prognosis has improved across all cancer stages over the last decade through improvements in surgical and medical oncological management. Targeted therapies with anti-epidermal growth factor receptor (EGFR) drugs (for example, cetuximab in RAS non-mutants) or anti-vascular endothelial growth factor (VEGF) treatment (for example, bevacizumab for RAS mutants) have become the standard of care1. However, a considerable number of patients treated with curative intent will develop metastases and eventually die of disseminated treatment-resistant disease. Effective chemotherapy regimens, combined with biological agents, have increased the median survival to > 3 years for patients with stage IV disease. Increased resection rates for hepatic metastasis are associated with 5-year overall survival approaching 50 per cent after liver surgery2, although only about 1 in 5 patients can be offered metastatic surgery. Despite the progress made, < 15 per cent of all patients with stage IV CRC are alive at 5 years from diagnosis3.
In parallel with improved management, the molecular pathways in CRC have been described in more detail, with proposed implication for personalized therapy4. Avoiding immune destruction is a cancer hallmark5 and is facilitated through several mechanisms, including KRAS mutations in CRC6. However, an immunogenic subtype of CRC has been found to be associated with a favourable outcome and a remarkable and durable response to immunotherapy7.
The immunogenic subtype of CRC is related to a defective mismatch repair (dMMR) system and associated with high-frequency microsatellite instability (MSI-H). The dMMR/MSI-H cancers have long been associated with strong lymphocytic infiltration in and around the tumour8,9. The dMMR tumours have a high tumour mutational burden and an abundance of neoantigens7,10, the latter contributing to an activated immune cell response and antitumour activity11–13. However, not all immune cells in the tumour microenvironment are activated against the tumour cell14, and immune escape is an essential part of clonal cancer evolution15,16. Metaphorically speaking, the cancer–immune cell interaction renders tumours either ‘immune cold’ or ‘immune altered’, or ‘immune hot’17, reflecting an opportunity for T cell activation by immune checkpoint blockade to promote cancer cell destruction (Fig. 1). While dichotomization of the immune response is unlikely to be biologically correct, there is emerging interest in mechanisms that can activate immune-dormant tumours. The dormant nature is usually attributed to exhausted T cells which may be activated by immune checkpoint inhibitors. While immune checkpoint inhibition is most effective in dMMR cancers18, the involved mechanisms are currently incompletely understood and may not be related to MMR status alone7. Indeed, some microsatellite-stable (MSS) cancers are hypermutated, based on specific genetic mutations such as polymerase epsilon (POLE) and polymerase delta (POLD1)19,20. POLE and POLD1 are proofreading domains of the DNA which repair genetic defects. Mutations in these genes are found in < 4 per cent of CRCs20. However, when present, POLE/POLD1-mutated cancers are associated with a high tumour mutational burden; hence, their use has also been suggested in predicting efficacy of immunotherapy19–22. Of note, recent studies have suggested that the rate of tumour mutational burden in MSS cancers has relevance to immunotherapy response, with a higher tumour mutational burden showing response to immunotherapy7,23–26. Several relevant genes have been associated with a high tumour mutational burden (including ARID1A, RNF43, BRAF and KM2B in microsatellite instability (MSI) cancers) and may help select MSS tumours for immunotherapy25–27. Some studies have also suggested using tumour mutational burden as a biomarker for measuring response to immunotherapy. Measuring tumour mutational burden in MSS cancers may be helpful in selecting patients who would benefit from immunotherapy. However, it is currently not clear which test works best for selecting patients suitable for immunotherapy. In a systematic review, concurrent occurrence of MSI, high tumour mutational burden and positive programmed cell death ligand 1 (PD-L1) expression was found in only 12.8 per cent of CRCs23. MSI and tumour mutational burden had the greatest overlap, with PD-L1 positive expression occurring in > 40 per cent of cases with no associated MSI or tumour mutational burden. Hence, relying on any one single test may not capture all patients who may eventually have a response and benefit from immunotherapy. Notably, most studies on treatment-refractory metastatic CRC have relied on dMMR subtypes for inclusion in trials28–30.
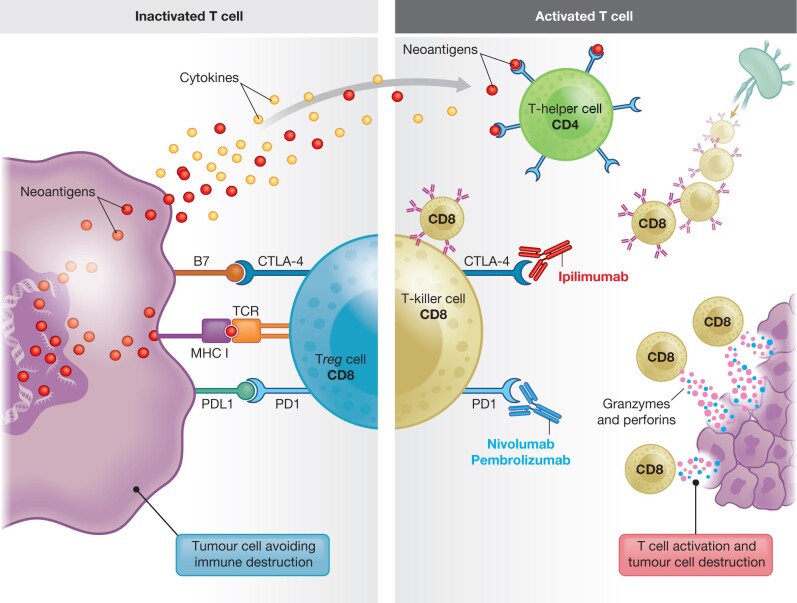
Fig. 1.
Immunotherapy by immune checkpoint blockage in colorectal cancer
Simplistic overview of mechanisms of T cell activation/inactivation in the tumour microenvironment. The inactive state of T cells in immune ‘cold’ tumours (on the left). Major histocompatibility complex (MHC)-T cell receptor (TCR)-dependent signalling demonstrates immune evasion by tumour cells expressing the inhibitory ligand PD-L1, which binds to PD-1 on T cells (and B7 molecules) which bind to CTLA-4. Cancer cell engagement with inhibitory ligands (against PD-1 and CTLA-4) prevents cytotoxic killing of tumour cells. PD-L1 binding initiates a signalling cascade that stimulates conversion of effector T cells to regulatory T cells (Tregs). Tumours in the active state (immune ‘hot’ tumours on right side of panel). CD8+ T cells recognize tumour-associated antigens expressed on MHC class I on tumour cells via the TCR, which results in cytotoxic killing of tumour cells via release of granzymes and perforins. Inhibitory ligands against PD-1 and CTLA-4 are blocked by immune checkpoint inhibitors, allowing active T cell function towards cancer cells. Several associated mechanisms are involved, in addition, including dendritic cells that serve as a biologic immune intermediate for neoantigen delivery, with an ability to augment the immune response through cytokine release. CTLA-4, cytotoxic T lymphocyte-associated protein 4; PD-1, programmed cell death 1; PD-L1, PD-1 ligand.
There is considerable interest in using immunotherapy beyond the metastatic setting, supported by the remarkable progress made in several other solid organ cancers with use of immune checkpoint inhibitors31. This review describes the potential influence of immunotherapy on operable and advanced CRC and possible related changes to clinical practice and research.
Mechanisms of action of immunotherapy in CRC with dMMR
The Food and Drug Administration (FDA) first approved the use of immunotherapy drugs for metastatic CRC in 201718,32–34. Recent trials have suggested a potential change in management of both operable and advanced CRC with use of immune checkpoint inhibitors35,36. The currently approved drugs nivolumab and pembrolizumab block the programmed cell death 1 (PD-1) receptor, whereas ipilimumab blocks cytotoxic T cell-associated protein 4 (CTLA-4) (Fig. 1). Several other tumour–immune cell interactions are important for immune system activation or depression in cancer, including major histocompatibility complex (MHC) class I interaction and signalling with the T cell receptor (TCR). Several of these tumour–immune cell interactions are under investigation for potential therapeutic use7,31,37. Recent data suggest that downregulation of MHC class II protein is associated with a poorer immune response and scant number of T cells surrounding the tumour, with an opportunity to activate an immune response through MHC class II38,39. Notably, the mechanisms underlying the tumour–immune cell environment are complex and include several possible co-evolving pathways along the adenoma–carcinoma–metastasis pathway in CRC11,15,40–42.
PD-1 and/or CTLA-4 checkpoint inhibition has proven to be highly effective for the treatment of patients with advanced dMMR CRC18,32. Patients with dMMR/MSI-H cancers have a high tumour mutational burden due to a very high number of missense mutations, including indels and frameshift mutations in the tumour genome. As a result, these genetic alterations produce a high number of neoantigens (or alternatively referred to as neopeptides or neoepitopes) that are tumour-specific and may be recognized by immune cells as foreign, hence eliciting an immune response with an abundance of T cells in the tumour surroundings. The abundance and type of immune cells are related to clinical outcome8, with better disease-specific survival rates in patients with a high number of tumour-infiltrating lymphocytes (TILs)9,43. However, in some cancers, T cells may be inactive due to binding of the PD-1 receptor to the tumour cell ligand PD-L1. In this instance, cytotoxic T cells are converted to regulatory T cells (Tregs) which do not exert a destructive effect on tumour cells. Through blockade of PD-1 (for example, pembrolizumab), cytotoxic T cells are activated and release cytotoxic signals that initiate cancer cell destruction (Fig. 1).
Immunotherapy according to consensus molecular subtypes
Classification based on consensus molecular subtypes (CMS)44 broadly defines disease into four groups (Fig. 2) with clinical relevance4,45–47. CMS type 1 (or ‘MSI immune’) is particularly suited for immunotherapy, based on the descriptive characteristics of such cancers18,48,49. Hence, testing for specific molecular traits and measuring immune cell infiltration have become important for both prognostic and predictive purposes in CRC9,50–52. For patients with localized CRC (stages I–III) with dMMR (usually around 15–20 per cent of all CRCs), overall prognosis is better than that for patients with proficient mismatch repair (pMMR) tumours9,53. However, dMMR CRC with metastasis has a very poor prognosis. Metastatic tumours with MSI/dMMR are most often driven by associated BRAF mutation54, and such metastatic dMMR tumours have poor response to chemotherapy and an overall worse prognosis55. Hence, molecular features such as MSI and KRAS and BRAF mutations have clear clinical implications and have become essential predictors beyond regular image-based tumour staging.
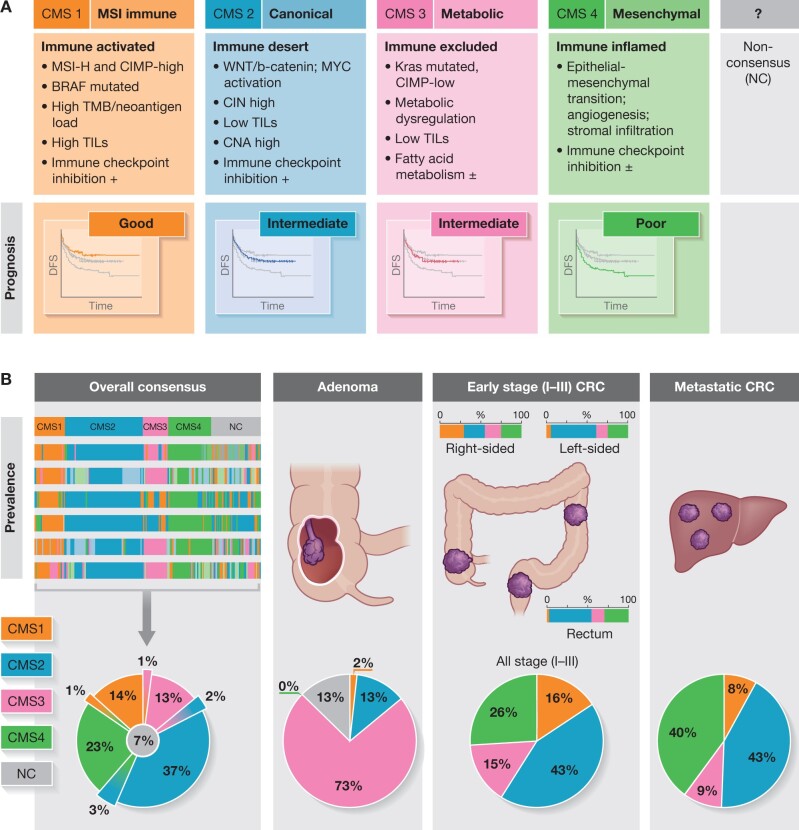
Fig. 2.
Context- and stage-based prevalence of consensus molecular subtypes in early-stage and advanced colorectal cancer
Depicted are the main features of each of the four subtypes (a) and the context-dependent distribution across disease stages (b). The actual CMS distribution may vary between trials and studies, based on inclusion criteria, with direct impact on study population outcomes. Data are approximated from several different studies.
However, as demonstrated in several subsequent studies, the CMS groups are not confined within stages and are context-driven in terms of CMS distribution across stage categories of CRC (Fig. 2b). Of note, the initial consensus series failed to categorize 7 per cent (with a further 6 per cent having mixed categories) of patients determined as ‘non-categorical’ (NC; Fig. 2)44. Furthermore, studies have shown that CMS distribution is variable across stages of carcinogenesis (Fig. 2b), with a low prevalence of CMS1 in adenomas56. A variable prevalence of CMS1 is found across clinical series in stages I–III, with about 16 per cent across the entire colorectum57, but with > 25 per cent prevalence for colon cancers and a corresponding lower rate in the rectum. In metastatic (stage IV) disease, a variable CMS1 rate of around 8 per cent has been reported58. The distribution of CMS categories largely varies between right- or left-sided colonic cancers and rectal cancers (Fig. 2b). Hence, the distribution of the ‘MSI immune’ type is more frequently found in right-sided colonic cancers (up to 25 per cent), but relatively rarely in metastatic CRC (about 8 per cent). Immunotherapy benefit is not solely related to CMS1. As demonstrated in Fig. 2, CMS2 and CMS3 generally show an intermediate response to immunotherapy, whereas CMS4 has a poor response. KRAS mutations are reported in < 15 per cent of liver metastases overall, yet about 25–40 per cent of patients with resected liver metastases harbour KRAS mutation59–61. Furthermore, MSI and mismatch repair deficiencies are found more frequently in young-onset CRC and in the elderly with CRC. It seems that rather than categorically placing patients into individual subtypes, the spectrum of immune cell responses demonstrates variation from primary to metastatic tumour62, with some being immune ‘hot’ and others ‘altered’ or ‘cold’ (Fig. 3)63. Therefore, the number of factors involved in immune-activated compared to those in immune-deserted cases show overlap and do not have a perfect correlation with mismatch repair testing or the presence of MSI or tumour mutational burden, nor with quantitative measurement of immune cells beyond group levels. Thus, this may be a slight impediment to perfect prognostic and predictive testing for personalized therapy in CRC. Of note, both dMMR and pMMR may have unexpected responses and failures64,65, which are not currently understood.
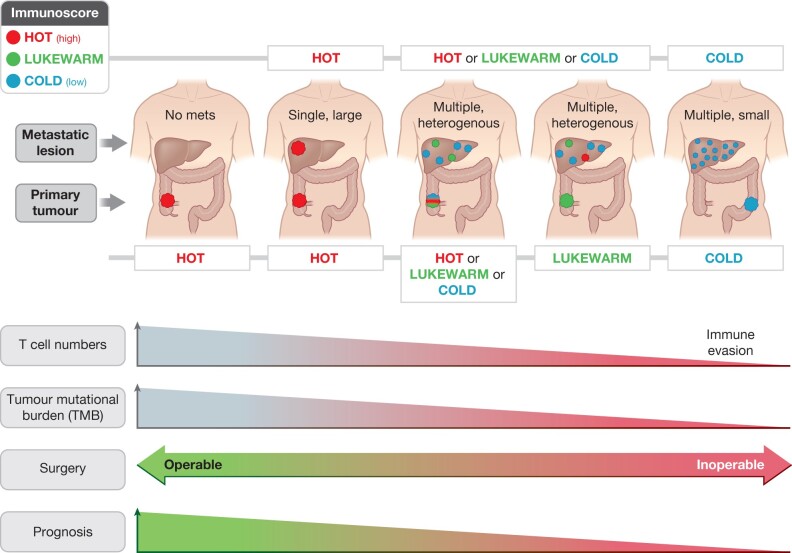
Fig. 3.
Variation in the immune landscape from early- to advanced-stage CRC
The model of immune ‘hot’ and ‘cold’ tumours across the spectrum of primary and stage IV disease is illustrated, based on63. Several other factors are involved, including age and sex of patient, tumour sidedness (with more dMMR in right-sided colon cancers being immune hot). Patterns of clinical scenarios associated with the immune profile are emerging such as association of multiple small disseminated liver metastases found in immune ‘cold’ tumours. The clinical presentation of CRC, together with mismatch repair status, immune cell quantification, and molecular features such as tumour mutational burden, may help delineate appropriate and personalized use of immunotherapy and the design of new trials.
Immunotherapy as first-line treatment in metastatic CRC
The efficacy of immunotherapy as second-line treatment in chemotherapy-refractory metastatic CRC was assessed in CheckMate 14232,33. The trial examined the combined effect of nivolumab and ipilimumab for dMMR metastatic CRC and showed high response rates, with progression-free and overall survival at 12 months at 71 per cent and 85 per cent, respectively. This combination treatment also proved to be safe in terms of efficacy. CheckMate 14232,33 therefore demonstrated that immunotherapy had a place in treating patients with mCRC and MSI-H/dMMR where other treatment no longer had an effect.
The randomized, open-label, multicentre KEYNOTE-177 trial64 has changed the standard of care for metastatic CRC with dMMR36. The study included 307 patients with metastatic CRC and MSI-H/dMMR. In this single-agent immunotherapy trial, progression-free survival was almost twice as long as that in the pembrolizumab group, compared to the chemotherapy group (median progression-free survival of 16.5 months for pembrolizumab versus 8.2 months for chemotherapy). Duration of response was also longer in the pembrolizumab group, with 84 per cent of patients still having partial or complete response 24 months after therapy initiation, versus 33 per cent of patients in the chemotherapy group. The complete response rate was also higher in the pembrolizumab group, compared to the chemotherapy group66. With fewer severe complications in the pembrolizumab group, safety of the drug was also demonstrated, but more immune-mediated adverse events were recorded, as expected. Therefore, this study shows pembrolizumab to be superior to chemotherapy for patients with MSI-H/dMMR metastatic CRC , with longer progression-free survival and fewer severe adverse events64.
Immunotherapy as upfront treatment in operable CRC
For operable colon cancer (stages I–III), traditional management has been surgery followed by adjuvant chemotherapy for those at high risk of relapse, typically high-risk stage II and all stage III colon cancer. In rectal cancer, neoadjuvant chemotherapy and radiotherapy are more frequently used to improve local control ahead of surgery. However, there has been recent interest in using neoadjuvant chemotherapy in colon cancer patients, particularly in those with locally advanced tumours67.
The large multicentre trial (FOxTROT68) investigating the role of neoadjuvant chemotherapy in high-risk colon cancer has not yet produced results on final outcomes, including survival data. Currently, the standard of care for operable stage I–III colon cancer is surgery upfront, with adjuvant therapy for patients with high-risk features. Little is known about immunotherapy in operable CRC, although a higher rate (around 25 per cent for colon cancer) of dMMR/MSI-H is expected in this setting, compared to metastatic CRC (dMMR reported to be 6–8 per cent) (Fig. 2). Hence, the prospect of a clinical benefit in operable colon cancer should be higher, particularly if guided by the relatively higher frequency of dMMR tumours. Also, rectal cancers with dMMR have a poor response to conventional radiochemotherapy52, with anecdotal reports of a very good response to immunotherapy69,70. One study in patients with rectal cancer investigated pretreatment biopsies and found an association between high tumour mutational burden and high T cell infiltration to have a better subsequent response to radiochemotherapy, possibly suggesting that adding immunotherapy may further enhance this effect71.
In the NICHE phase I/II trial65, the effect of neoadjuvant immunotherapy by doublet immune check blockade was investigated in a cohort of 40 patients with operable colon cancer. Both MSI-H/dMMR (21 patients) and MSS/pMMR (20 patients) cancers were included, of which 35 were evaluable for efficacy and translational endpoints (20 dMMR and 15 pMMR). Patients were given double immune checkpoint blockade with a single dose of ipilimumab and two doses of nivolumab 6 weeks prior to surgery. The treatment was well tolerated and all patients underwent radical resections without delay (meeting the primary endpoint of the trial). Pathological response was observed in 20/20 of dMMR tumours, with 19 major pathological responses (defined as ≤ 10 per cent residual viable tumour on histopathology) and 12 (60 per cent) pathological complete responses. Notable in the NICHE trial, among the pMMR tumours, 4 (3 major and 1 partial responses) of 15 had pathological responses65. The difference in response between dMMR and pMMR is mainly attributed to a difference in tumour burden/neoantigens and T cell infiltration. Notably, CD8+PD-1+ T cell infiltration was predictive of response in pMMR tumours, suggesting that some pMMR tumours are immune-responsive despite not demonstrating dMMR at the molecular level. This demonstrates the complexity of defining ‘hot’ and ‘cold’ tumours with response to therapy beyond simple MMR testing (Fig. 3)63.
The NICHE-1 study data indicate that neoadjuvant immunotherapy has the potential to become the standard of care for a defined group of colon cancer patients when validated in larger studies. The phase I/II results are corroborated by early reports in rectal cancer with proven MSI-H69,70. The NICHE study also points to an important issue in patient selection beyond dMMR status. One could perceive that absolute T cell counts, or selection by immunoscore, tumour mutational burden analyses, or PD-L1 assessment, may be warranted to identify those patients who would likely benefit from immunotherapy in pMMR cancers. However, correlation among such tests, actual treatment response and effect on overall survival is not yet established. Lastly, one should also question reflexive treatment for all operable dMMR CRC, as a vast majority of these patients will never have recurrence and hence would not benefit from treatment, even if a complete response is obtained. In the setting where resection is planned, the prospects of downstaging in large or bulky tumours may be the most likely benefit of neoadjuvant immunotherapy. However, one could potentially take advantage of the complete response observed in selected patients having non-operative, surveillance-based management, to allow an organ-sparing approach. This would be analogous to the current watch-and-wait trials after complete pathological response to neoadjuvant chemoradiotherapy in rectal cancer72,73.
Resistance and sustainable effects
Despite the demonstrated effects of immunotherapy, there are concerns regarding immune checkpoint blockade. One concern relates to durability of response and the development of immunotherapy resistance74. A hallmark of immunotherapy is presentation of an antigen that the host’s T cell can identify as an intruder cell, and thus initiation of destruction. Hence, immunotherapy resistance can develop from alteration in tumour cells’ lack or ineffective presentation of antigens. Other mechanisms of resistance include aberrant cellular signal transduction and changes in cellular components in the tumour microenvironment, both influencing T cell response to anti-PD-1 therapy. With CTLA-4 blockade resistance, different mechanisms are described. CTLA-4 immune checkpoint is dependent on the costimulatory molecule B7 to induce a response and T cells mediate their effect on CTLA-4 blockade through interferon gamma. Tumour cells develop resistance through changing the expression of the costimulatory molecule B7 oraltered genes which respond to interferon gamma signalling initiated by T cells7,74.
Recently, reports that fecal microbiota transplantation can lead to immune checkpoint inhibitor (ICI) therapy responses in patients previously refractory to therapy suggest that targeting the microbiome may be a viable strategy to reprogramme the tumour microenvironment and augment immunotherapy75. A small study76 in treatment-refractory melanoma demonstrated the effect of fecal microbiota transplantation in changing the gut flora, and hence reprogramming the tumour microenvironment, to make the cancer more immunogenic. Whether this principle is transferable to other cancers, such as CRC, is currently under investigation.
Adverse events to immune checkpoint inhibitors
Adverse events from immunotherapy are important as they can increase morbidity, delay surgery in the neoadjuvant setting, and, in worst case scenarios, increase mortality. One review described the colon, liver, lungs, pituitary, thyroid, skin, and, more rarely, the heart and nervous system as affected organs. With ipilimumab (anti-CTLA-4) monotherapy, the most common adverse effect is colitis, whereas nivolumab and pembrolizumab (anti-PD-1) have been found to cause hypothyroidism, rash, and diarrhoea (more commonly than colitis). Combination treatment with both anti-CTLA-4 and anti-PD-1 carry the highest risk of adverse effects, particularly colitis, and often affected multiple organs.
Early concern about immunotherapy-induced colitis preventing subsequent surgery seems unwarranted, with very low numbers (< 1–5 per cent) found across 145 trials77, of which only three were conducted in patients with CRC. A slightly higher risk may be found in inflammatory bowel disease-associated cancers, but none have been reported to have treatment-limiting effects78. Fatal toxic event rates are very low for ICI (between 0.3 and 1.3 per cent), but with the vast majority of data coming from studies in patients with end-stage melanoma or lung cancer79.
Future direction
Immunotherapy has proven to be highly effective in CRC, with excellent results as first-line treatment in the metastatic setting for dMMR CRC using monotherapy pembrolizumab. Further, the high response rate in operable dMMR colon cancers with preoperative use of double nivolumab and ipilimumab therapy warrants further investigation for its impact on long-term overall survival.
New opportunities for immunotherapy are emerging (Fig. 3) that are likely to exploit the immune system to enhance multimodal therapy, improve resection rates, enhance disease control, and eventually improve overall survival for patients. With this, several questions arise with new avenues for research and trials. The current understanding of what makes tumours ‘hot’ and ‘cold’ is incomplete, although data are emerging17. The criteria for potential treatment beyond MMR/MSI testing and POLE/POLD1 are unclear, but data from the NICHE trial suggest that immunotherapy may have the potential for complete response in dMMR tumours and at least partial response in some pMMR tumours with higher levels of CD8+ T cells65.
Currently, the optimal timing and strategy for immunotherapy use are uncertain. Should the immune system be modulated as early as possible to potentially achieve the best oncological results? It is feasible that neoadjuvant immune checkpoint blockade could become the preferred option in operable CRC, if this translates into reduced recurrence and improved overall survival. It is possible that immunotherapy may become an organ-sparing treatment option, if complete response is durable and sustained and translates into equivalent oncological effects to surgery. However, there are currently no data to suggest which patients in stage I–III CRC should benefit from this. Hence, novel criteria for appropriate selection for use of immune checkpoint inhibitors must be developed to show the effects on survival outcomes in larger trials. For metastatic disease, it is obviously of interest to explore the ability of immune checkpoint inhibitors to facilitate conversion from unresectable to resectable disease and to improve survival2. Further, in what way immunotherapy belongs to just one or more of the CMS subtypes in CRC is not clear. Further research should therefore explore the clinical impact of immunotherapy in both early-stage and advanced CRC.
Acknowledgement
A.K. and T.V. contributed equally.
Funding
This research was funded in part by the Folke Hermansen Cancer Fund and an unrestricted grant from Stavanger University Hospital.
Disclosure. The authors declare no conflict of interest.
References
- 1. Messersmith WA. NCCN guidelines updates: management of metastatic colorectal cancer. J Natl Compr Canc Netw 2019;17:599–601. [DOI] [PubMed] [Google Scholar]
- 2. Kopetz S, Chang GJ, Overman MJ, Eng C, Sargent DJ, Larson DW. et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol 2009;27:3677–3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stebbing J, Singh Wasan H.. Decoding metastatic colorectal cancer to improve clinical decision making. J Clin Oncol 2019;37:1847–1850. [DOI] [PubMed] [Google Scholar]
- 4. Dienstmann R, Vermeulen L, Guinney J, Kopetz S, Tejpar S, Tabernero J.. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat Rev Cancer 2017;17:79–92. [DOI] [PubMed] [Google Scholar]
- 5. Hanahan D, Weinberg RA.. Hallmarks of cancer: the next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 6. Liao W, Overman MJ, Boutin AT, Shang X, Zhao D, Dey P. et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 2019;35:559–572.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lizardo DY, Kuang C, Hao S, Yu J, Huang Y, Zhang L.. Immunotherapy efficacy on mismatch repair-deficient colorectal cancer: from bench to bedside. Biochim Biophys Acta Rev Cancer 2020;1874:188447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pagès C. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 9. Watson MM, Lea D, Gudlaugsson E, Skaland I, Hagland HR, Søreide K.. Prevalence of PD-L1 expression is associated with EMAST, density of peritumoral T-cells and recurrence-free survival in operable non-metastatic colorectal cancer. Cancer Immunol Immunother 2020;69:1627–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Westdorp H, Fennemann FL, Weren RD, Bisseling TM, Ligtenberg MJ, Figdor CG. et al. Opportunities for immunotherapy in microsatellite instable colorectal cancer. Cancer Immunol Immunother 2016;65:1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Roudko V, Bozkus CC, Orfanelli T, McClain CB, Carr C, O’Donnell T. et al. Shared immunogenic poly-epitope frameshift mutations in microsatellite unstable tumors. Cell 2020;183:1634–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ballhausen A, Przybilla MJ, Jendrusch M, Haupt S, Pfaffendorf E, Seidler F. et al. The shared frameshift mutation landscape of microsatellite-unstable cancers suggests immunoediting during tumor evolution. Nat Commun 2020;11:4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Germano G, Lamba S, Rospo G, Barault L, Magrì A, Maione F. et al. Inactivation of DNA repair triggers neoantigen generation and impairs tumour growth. Nature 2017;552:116–120. [DOI] [PubMed] [Google Scholar]
- 14. Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW. et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557:575–579. [DOI] [PubMed] [Google Scholar]
- 15. Angelova M, Mlecnik B, Vasaturo A, Bindea G, Fredriksen T, Lafontaine L. et al. Evolution of metastases in space and time under immune selection. Cell 2018;175:751–765.e16. [DOI] [PubMed] [Google Scholar]
- 16. Woolston A, Khan K, Spain G, Barber LJ, Griffiths B, Gonzalez-Exposito R. et al. Genomic and transcriptomic determinants of therapy resistance and immune landscape evolution during anti-EGFR treatment in colorectal cancer. Cancer Cell 2019;36:35–50.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Galon J, Bruni D.. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discov 2019;18:197–218. [DOI] [PubMed] [Google Scholar]
- 18. Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med 2015;372:2509–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Picard E, Verschoor CP, Ma GW, Pawelec G.. Relationships between immune landscapes, genetic subtypes and responses to immunotherapy in colorectal cancer. Front Immunol 2020;11:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. He J, Ouyang W, Zhao W, Shao L, Li B, Liu B. et al. Distinctive genomic characteristics in POLE/POLD1-mutant cancers can potentially predict beneficial clinical outcomes in patients who receive immune checkpoint inhibitor. Ann Transl Med 2021;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Budczies J, Seidel A, Christopoulos P, Endris V, Kloor M, Győrffy B. et al. Integrated analysis of the immunological and genetic status in and across cancer types: impact of mutational signatures beyond tumor mutational burden. Oncoimmunology 2018;7:e1526613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yao J, Gong Y, Zhao W, Han Z, Guo S, Liu H. et al. Comprehensive analysis of POLE and POLD1 gene variations identifies cancer patients potentially benefit from immunotherapy in Chinese population. Sci Rep 2019;9:15767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T. et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol 2019;30:1232–1243. [DOI] [PubMed] [Google Scholar]
- 24. Schrock AB, Ouyang C, Sandhu J, Sokol E, Jin D, Ross JS. et al. Tumor mutational burden is predictive of response to immune checkpoint inhibitors in MSI-high metastatic colorectal cancer. Ann Oncol 2019;30:1096–1103. [DOI] [PubMed] [Google Scholar]
- 25. Fabrizio DA, George TJ Jr, Dunne RF, Frampton G, Sun J, Gowen K. et al. Beyond microsatellite testing: assessment of tumor mutational burden identifies subsets of colorectal cancer who may respond to immune checkpoint inhibition. J Gastrointest Oncol 2018;9:610–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bao X, Zhang H, Wu W, Cheng S, Dai X, Zhu X. et al. Analysis of the molecular nature associated with microsatellite status in colon cancer identifies clinical implications for immunotherapy. J Immunother Cancer 2020;8:e001437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mehrvarz Sarshekeh A, Alshenaifi J, Roszik J, Manyam G, Advani SM, Katkhuda R. et al. ARID1A mutation may define an immunologically active subgroup in patients with microsatellite-stable colorectal cancer. Clin Cancer Res 2021;27:1663–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Segal NH, Cercek A, Ku GY, Wu AJ, Rimner A, Khalil DN. et al. Phase II single arm study of durvalumab and tremelimumab with concurrent radiotherapy in patients with mismatch repair proficient metastatic colorectal cancer. Clin Cancer Res 2021;27:2200–2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Le DT, Kim TW, Van Cutsem E, Geva R, Jäger D, Hara H. et al. Phase II open-label study of pembrolizumab in treatment-refractory, microsatellite instability-high/mismatch repair-deficient metastatic colorectal cancer: KEYNOTE-164. J Clin Oncol 2020;38:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen EX, Jonker DJ, Loree JM, Kennecke HF, Berry SR, Couture F. et al. Effect of combined immune checkpoint inhibition vs best supportive care alone in patients with advanced colorectal cancer: The Canadian Cancer Trials Group CO.26 study. JAMA Oncol 2020;6:831–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Meric-Bernstam F, Larkin J, Tabernero J, Bonini C.. Enhancing anti-tumour efficacy with immunotherapy combinations. Lancet 2021;397:1010–1022. [DOI] [PubMed] [Google Scholar]
- 32. Overman MJ, Lonardi S, Wong KYM, Lenz HJ, Gelsomino F, Aglietta M. et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability-high metastatic colorectal cancer. J Clin Oncol 2018;36:773–779. [DOI] [PubMed] [Google Scholar]
- 33. Overman MJ, McDermott R, Leach JL, Lonardi S, Lenz HJ, Morse MA. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol 2017;18:1182–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marcus L, Lemery SJ, Keegan P, Pazdur R.. FDA approval summary: pembrolizumab for the treatment of microsatellite instability-high solid tumors. Clin Cancer Res 2019;25:3753–3758. [DOI] [PubMed] [Google Scholar]
- 35. Coukos G. Neoadjuvant immune-checkpoint blockade in resectable colon cancer. Nat Med 2020;26:473–474. [DOI] [PubMed] [Google Scholar]
- 36. Grothey A. Pembrolizumab in MSI-H-dMMR advanced colorectal cancer – a new standard of care. N Engl J Med 2020;383:2283–2285. [DOI] [PubMed] [Google Scholar]
- 37. Thomas J, Leal A, Overman MJ.. Clinical development of immunotherapy for deficient mismatch repair colorectal cancer. Clin Colorectal Cancer 2020;19:73–81. [DOI] [PubMed] [Google Scholar]
- 38. Dunne MR, Phelan JJ, Michielsen AJ, Maguire AA, Dunne C, Martin P. et al. Characterising the prognostic potential of HLA-DR during colorectal cancer development. Cancer Immunol Immunother 2020;69:1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Luo Z, Chen X, Zhang Y, Huang Z, Zhao H, Zhao J. et al. Development of a metastasis-related immune prognostic model of metastatic colorectal cancer and its usefulness to immunotherapy. Front Cell Dev Biol 2020;8:577125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Willis JA, Reyes-Uribe L, Chang K, Lipkin SM, Vilar E.. Immune activation in mismatch repair-deficient carcinogenesis: more than just mutational rate. Clin Cancer Res 2020;26:11–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bergholz JS, Wang Q, Kabraji S, Zhao JJ.. Integrating immunotherapy and targeted therapy in cancer treatment: mechanistic insights and clinical implications. Clin Cancer Res 2020;26:5557–5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ganesh K, Stadler ZK, Cercek A, Mendelsohn RB, Shia J, Segal NH. et al. Immunotherapy in colorectal cancer: rationale, challenges and potential. Nat Rev Gastroenterol Hepatol 2019;16:361–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Watson MM, Kanani A, Lea D, Khajavi RB, Søreide JA, Kørner H. et al. Elevated Microsatellite Alterations at Selected Tetranucleotides (EMAST) in colorectal cancer is associated with an elderly, frail phenotype and improved recurrence-free survival. Ann Surg Oncol 2020;27:1058–1067. [DOI] [PubMed] [Google Scholar]
- 44. Guinney J, Dienstmann R, Wang X, de Reyniès A, Schlicker A, Soneson C. et al. The consensus molecular subtypes of colorectal cancer. Nat Med 2015;21:1350–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Smeby J, Sveen A, Merok MA, Danielsen SA, Eilertsen IA, Guren MG. et al. CMS-dependent prognostic impact of KRAS and BRAFV600E mutations in primary colorectal cancer. Ann Oncol 2018;29:1227–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lenz HJ, Ou FS, Venook AP, Hochster HS, Niedzwiecki D, Goldberg RM. et al. Impact of consensus molecular subtype on survival in patients with metastatic colorectal cancer: results from CALGB/SWOG 80405 (Alliance). J Clin Oncol 2019;37:1876–1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Berg KCG, Sveen A, Høland M, Alagaratnam S, Berg M, Danielsen SA. et al. Gene expression profiles of CMS2-epithelial/canonical colorectal cancers are largely driven by DNA copy number gains. Oncogene 2019;38:6109–6122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bruni D, Angell HK, Galon J.. The immune contexture and immunoscore in cancer prognosis and therapeutic efficacy. Nat Rev Cancer 2020;20:662–680. [DOI] [PubMed] [Google Scholar]
- 49. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK. et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357:409–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Angell HK, Bruni D, Barrett JC, Herbst R, Galon J.. The immunoscore: colon cancer and beyond. Clin Cancer Res 2020;26:332–339. [DOI] [PubMed] [Google Scholar]
- 51. Pagès F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C. et al. International validation of the consensus immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128–2139. [DOI] [PubMed] [Google Scholar]
- 52. Cercek A, Dos Santos Fernandes G, Roxburgh CS, Ganesh K, Ng S, Sanchez-Vega F. et al. Mismatch repair-deficient rectal cancer and resistance to neoadjuvant chemotherapy. Clin Cancer Res 2020;26:3271–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou R, Zhang J, Zeng D, Sun H, Rong X, Shi M. et al. Immune cell infiltration as a biomarker for the diagnosis and prognosis of stage I-III colon cancer. Cancer Immunol Immunother 2019;68:433–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Venderbosch S, Nagtegaal ID, Maughan TS, Smith CG, Cheadle JP, Fisher D. et al. Mismatch repair status and BRAF mutation status in metastatic colorectal cancer patients: a pooled analysis of the CAIRO, CAIRO2, COIN, and FOCUS studies. Clin Cancer Res 2014;20:5322–5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wensink GE, Elferink MAG, May AM, Mol L, Hamers PAH, Bakker SD. et al. Survival of patients with deficient mismatch repair metastatic colorectal cancer in the pre-immunotherapy era. Br J Cancer 2021;124:399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Komor MA, Bosch LJ, Bounova G, Bolijn AS, Delis-van Diemen PM, Rausch C. et al. ; NGS-ProToCol Consortium. Consensus molecular subtype classification of colorectal adenomas. J Pathol 2018;246:266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Coebergh van den Braak RRJ, Ten Hoorn S, Sieuwerts AM, Tuynman JB, Smid M, Wilting SM. et al. Interconnectivity between molecular subtypes and tumor stage in colorectal cancer. BMC Cancer 2020;20:850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Fontana E, Eason K, Cervantes A, Salazar R, Sadanandam A.. Context matters – consensus molecular subtypes of colorectal cancer as biomarkers for clinical trials. Ann Oncol 2019;30:520–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kamphues C, Andreatos N, Kruppa J, Buettner S, Wang J, Sasaki K. et al. The optimal cut-off values for tumor size, number of lesions, and CEA levels in patients with surgically treated colorectal cancer liver metastases: an international, multi-institutional study. J Surg Oncol 2021;123:939–948. [DOI] [PubMed] [Google Scholar]
- 60. Brudvik KW, Kopetz SE, Li L, Conrad C, Aloia TA, Vauthey JN.. Meta-analysis of KRAS mutations and survival after resection of colorectal liver metastases. Br J Surg 2015;102:1175–1183. [DOI] [PubMed] [Google Scholar]
- 61. Phipps AI, Alwers E, Harrison T, Banbury B, Brenner H, Campbell PT. et al. Association between molecular subtypes of colorectal tumors and patient survival, based on pooled analysis of 7 international studies. Gastroenterology 2020;158:2158–2168.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Van den Eynde M, Mlecnik B, Bindea G, Fredriksen T, Church SE, Lafontaine L. et al. The link between the multiverse of immune microenvironments in metastases and the survival of colorectal cancer patients. Cancer Cell 2018;34:1012–1026.e3 [DOI] [PubMed] [Google Scholar]
- 63. de Andrea CE, Schalper KA, Sanmamed MF, Melero I.. Immunodivergence in metastatic colorectal cancer. Cancer Cell 2018;34:876–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. André T, Shiu KK, Kim TW, Jensen BV, Jensen LH, Punt C. et al. ; KEYNOTE-177 Investigators. Pembrolizumab in microsatellite-instability-high advanced colorectal cancer. N Engl J Med 2020;383:2207–2218. [DOI] [PubMed] [Google Scholar]
- 65. Chalabi M, Fanchi LF, Dijkstra KK, Van den Berg JG, Aalbers AG, Sikorska K. et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–576. [DOI] [PubMed] [Google Scholar]
- 66. Ludford K, Cohen R, Svrcek M, Foo WC, Colle R, Parc Y. et al. Pathological tumor response following immune checkpoint blockade for deficient mismatch repair advanced colorectal cancer. J Natl Cancer Inst 2021;113:208–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Cheong CK, Nistala KRY, Ng CH, Syn N, Chang HSY, Sundar R. et al. Neoadjuvant therapy in locally advanced colon cancer: a meta-analysis and systematic review. J Gastrointest Oncol 2020;11:847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Seymour MT, Morton D; on behalf of the International FOxTROT Trial Investigators. FOxTROT: an international randomised controlled trial in 1052 patients (pts) evaluating neoadjuvant chemotherapy (NAC) for colon cancer. J Clin Oncol 2019;37:3504–3504. [Google Scholar]
- 69. Mans L, Pezzullo M, D’Haene N, Van de Stadt J, Van Laethem JL.. Pathological complete response after neoadjuvant immunotherapy for a patient with microsatellite instability locally advanced rectal cancer: should we adapt our standard management for these patients? Eur J Cancer 2020;135:75–77. [DOI] [PubMed] [Google Scholar]
- 70. Demisse R, Damle N, Kim E, Gong J, Fakih M, Eng C. et al. Neoadjuvant immunotherapy-based systemic treatment in MMR-deficient or MSI-high rectal cancer: case series. J Natl Compr Canc Netw 2020;18:798–804. [DOI] [PubMed] [Google Scholar]
- 71. Akiyoshi T, Tanaka N, Kiyotani K, Gotoh O, Yamamoto N, Oba K. et al. Immunogenomic profiles associated with response to neoadjuvant chemoradiotherapy in patients with rectal cancer. Br J Surg 2019;106:1381–1392. [DOI] [PubMed] [Google Scholar]
- 72. Fernandez LM, São Julião GP, Figueiredo NL, Beets GL, van der Valk MJM, Bahadoer RR. et al. ; International Watch & Wait Database Consortium. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol 2021;22:43–50. [DOI] [PubMed] [Google Scholar]
- 73. Wang QX, Zhang R, Xiao WW, Zhang S, Wei MB, Li YH. et al. The watch-and-wait strategy versus surgical resection for rectal cancer patients with a clinical complete response after neoadjuvant chemoradiotherapy. Radiat Oncol 2021;16:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Barrueto L, Caminero F, Cash L, Makris C, Lamichhane P, Deshmukh RR.. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol 2020;13:100738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hayase E, Jenq RR.. Role of the intestinal microbiome and microbial-derived metabolites in immune checkpoint blockade immunotherapy of cancer. Genome Med 2021;13:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM. et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021;371:595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ouyang T, Cao Y, Kan X, Chen L, Ren Y, Sun T. et al. Treatment-related serious adverse events of immune checkpoint inhibitors in clinical trials: a systematic review . Front Oncol 2021;11: 621639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sleiman J, Wei W, Shah R, Faisal MS, Philpott J, Funchain P.. Incidence of immune checkpoint inhibitor-mediated diarrhea and colitis (imDC) in patients with cancer and preexisting inflammatory bowel disease: a propensity score-matched retrospective study. J Immunother Cancer 2021;9:e002567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F. et al. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA Oncol 2018;4:1721–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]