Abstract
To explore the clinical features, imaging findings, and pathological manifestations of ovarian Sertoli-Leydig cell tumors (SLCTs). The clinical and pathological manifestations, tumor location, size, morphology, vascularity, computed tomography (CT) density, magnetic resonance imaging (MRI) signal intensity, and contrast enhancement patterns in five cases with SLCTs were retrospectively reviewed. SLCTs most commonly occurred in young women. Virilization was observed in three cases (60%). All five tumors were unilateral and oval or round, with a clear boundary. The solid part of the tumor was isoattenuated on the conventional CT scan, and showed isoattenuation or slight hypoattenuation relative to adjacent myometrium on T1 weighted imaging (T1WI) and T2 weighted imaging (T2WI). On contrast-enhanced images, three tumors showed marked enhancement. DICER1 hotspot mutations were commonly seen in SLCTs. A highly vascularized mass with low signal intensity (SI) of the solid part on T2WI and androgen overproduction symptoms may suggest an SLCT.
Keywords: Sertoli-leydig cell tumors, CT, MRI, pathology, ovary
Clinical practice points
SLCT is a rare type of sex-cord stromal tumor of ovary that most commonly occurs in young patients.
We retrospectively reviewed five cases of SLCT, Their clinical manifestations and imaging and imaging appearances were described and clinicopathological features were also evaluated.
A diagnosis of SLCT should be considered according to the combination of clinical manifestations, imaging features, and histologic appearances.
Testing for germline DICER1 mutations should be performed in all patients with ovarian SLCTs.
Introduction
Sertoli-Leydig cell tumors (SLCTs) are a rare type of ovarian sex cord stromal tumors, accounting for less than 0.5% of all primary ovarian neoplasms. 1 SLCTs can occur in all age groups, but are most frequently detected in young women with an average age of 25 years. 2 Hormone secretion of SLCTs is the reason for clinical symptoms including irregular menstruation and vaginal bleeding. Besides, the tumor mass may also lead to abdominal pain and swelling. Previous studies mainly focused on the clinicopathological features of SLCTs.3–5
The imaging series study made by Cai et al. 6 is the largest one of its kind, which showed that SLCTs were solid or multilocular cystic. Solid SLCTs usually appeared as low intensity on T1WI and intense enhancement on contrast-enhanced imaging. Multilocular cystic SLCTs often had thickened walls and septa. Kommoss and Lehr 1 reported that mutation analysis of DICER1 might be conducive to the differential diagnosis of SLCTs. However, to the best of our knowledge, the imaging characteristics and gene mutation features of SLCTs were rarely investigated simultaneously. In this study, we retrospectively reviewed the imaging and clinicopathological features of SLCTs in five cases, hoping to provide some clues for accurate diagnosis of SLCTs.
Patients
This retrospective study was approved by the institutional review boards of our hospital. Five patients diagnosed with ovarian SLCTs between 2010 and 2016 were included, and all of them had undergone imaging and histopathological examinations.
Imaging examination
CT scans were conducted with a Brilliance 64-slice helical CT scanner (Philips) at a layer thickness of 5 mm, a pitch of 1,120 kV tube voltage and a tube current of 200–500 mAs. MRI scanning was carried out with a SIEMENS 3.0T MRI system, and Gd-DTPA was used for MRI enhancement. The location, size, shape, boundary, components (solid, cystic, or mixed), and CT density or MRI signal intensity of ovarian lesions as well as abdominal and pelvic ascites of the patients were analyzed.
Pathologic examination
All five cases underwent the surgical resection, and pathological specimens were detected by standard Hematoxylin-eosin staining. Immunohistochemical staining was carried out in three cases. A pathologist reviewed and selected the relevant tissue from the tumor. DICER1 sequencing was performed on the original tumor tissue or the paraffin-embedded samples using either the Sanger method or the next generation sequencing technique.
Results
Clinical data
The clinical data are shown in Table 1. Patients’ age ranged from 17 to 60 (mean, 33.6 years old). Irregular menstruation and hirsutism were the main complaints of all three young patients (16, 17, and 21 years old, respectively). Enlargement of the clitoris was found in these young patients during physical examination, and one of them presented with deepening of the voice. Postmenopausal vaginal bleeding was the main symptom of the two old patients (54 and 60 years old, respectively). Three patients showed an elevated serum testosterone level. The tumor markers results revealed slightly raised carcinoembryonic antigen (CEA) in only one case.
Table 1.
The clinical manifestations of five cases of SLCT.
| Case | Age | Clinical features | T | Pathologicaldifferentiation | IHC results | DICER1 | Protein change |
|---|---|---|---|---|---|---|---|
| 1 | 17 | Irregularmenstruationvirilization | 647.72 | Intermediatedifferentiation | Vimentin | Mutation | c.5113G>A,p.E1705K |
| 2 | 21 | Irregularmenstruationvirilization | 317.46 | Intermediate—poordifferentiation | Inhibin-avimentin | Wild-type | / |
| 3 | 16 | Virilization | 77.5 | Intermediatedifferentiation | Inhibin-avimentin | Wild-type | / |
| 4 | 54 | Postmenopausalbleeding | 5.71 | Intermediatedifferentiation | / | / | / |
| 5 | 60 | Postmenopausalbleeding | 1.58 | Intermediate—poordifferentiation | Inhibin-acalretinin | Mutation | c.5113G>A,p.E1705K |
T: testosterone (normal, 0.15–0.5 ng/ml); IHC: immunohistochemical.
CT and MRI findings
The imaging features are displayed in Table 2. Five tumors were all unilateral and either oval or round, with a clear boundary. The size of these masses ranged from 3 cm to 30 cm.
Table 2.
Imaging findings of five cases of SLCT.
| Case | Site | Components | Size (cm) | Shape | Imagingmodality | CT density or MRIsignal intensityof the solid part |
|---|---|---|---|---|---|---|
| 1 | Right | Mainly cystic | 5.7 × 4.7 | Oval | CT | Low density |
| 2 | Right | Mainly solid | 4 × 3.6 | Round | CT/MRI | Heterogeneous density,hypointensity on T1WIand hyperintensity onT2WI, intense enhancement |
| 3 | Right | Mainly solid | 30 × 17 | Round | CT | Moderate density |
| 4 | Right | Mainly solid | 8 × 7 | Oval | CT | Moderate density |
| 5 | Left | Mainly cystic | 101 × 73 | Lobulated | CT | Intense septal enhancement |
Solid masses with patchy edema areas were found in two cases, and the other three cases had cystic masses with linear septa. Unenhanced CT values of solid parts of these masses ranged from 31 HU to 69 HU with an average of 34.2 ± 10.8 HU. The solid part of the tumor exhibited isoattenuation on the conventional CT scan, and isointensity or slight hypointensity relative to adjacent myometrium on T1WI and T2WI. Edema or cystic parts of the tumor showed low intensity on CT scanning (Figure 1(a)), hypointensity on T1WI and hyperintensity on T2WI (Figure 1(b) and (c)). Solid components were intensely enhanced while edema areas were slightly enhanced.
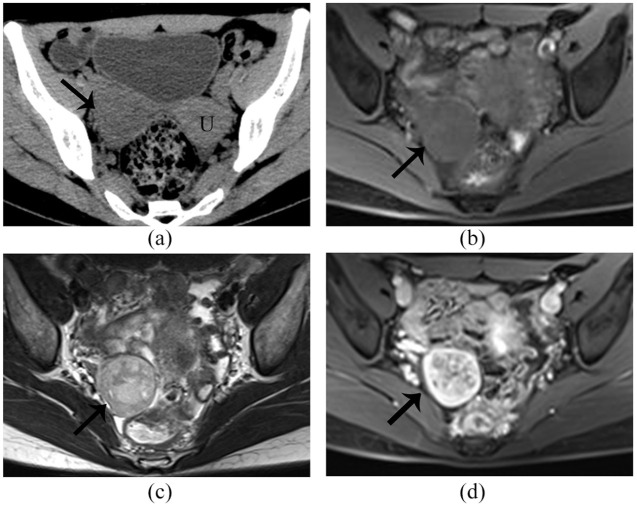
Figure 1.
Right ovarian SLCT in a 21-year-old woman. The tumor (arrow) shows low attenuation on (a) CT plain scan; magnetic resonance imaging, demonstrating a predominant solid mass in the right ovary. The tumor exhibits homogeneous isointense on (b) T1WI and slight heterogeneous isointense, with few patchy hyperintensity areas on the (c) T2WI. On contrast-enhanced images (d). The cystic and edema areas of the tumor exhibit no enhancement, whereas the solid part exhibits marked enhancement. U: uterus.
There were no positive results about the surrounding organs such as the uterus, ovary, or bowels. No enlarged lymph nodes or ascites were observed in all cases.
Pathologic examination
One tumor was a solid mass with patchy edema areas, while the remaining four cases had solid-cystic masses with different proportions of solid and cystic parts. These manifestations conformed to their imaging findings.
According to the histopathologic examination, the tumors were composed of ill-defined tubules formed by varying amounts of spindle-shaped Sertoli cells and delicate fibrous stroma containing clusters of Leydig cells. (Figure 2(a)).
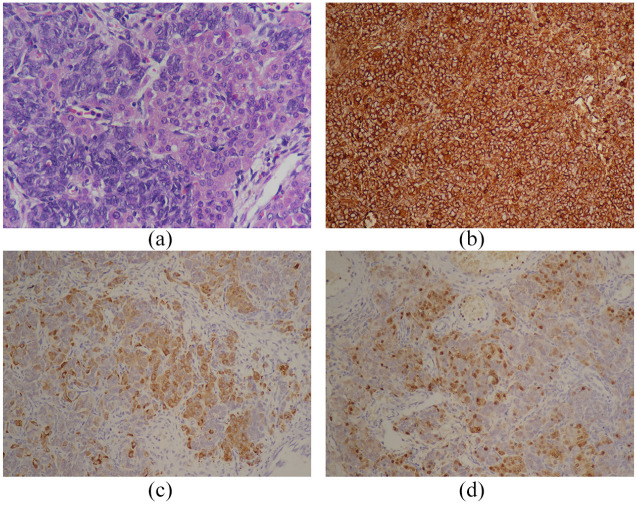
Figure 2.
Histological features of SLCT. (a) Hematoxylin ematoxylin and eosin staining (magnification, ×400), revealing bundles of spindle-shaped Sertoli cells forming ill-defined tubules and clusters of Leydig cells. Immunochemical examination showed that the tumor was positive for (b) vimentin (magnification, ×200), (c) inhinbin-a (magnification, ×200), and (d) calretinin (magnification, ×200).
Immunohistochemical staining was performed in four cases. Two cases were positively stained by Vimentin and Inhibin-a (Figure 2(b) and (c)), one by Vimentin, and one by calretinin (Figure 2(d)) and Inhibin-a. Somatic DICER1 mutations were identified in two patients, and two cases were wild-type mutations. One patient was not tested because its tumor specimen lost.
Discussion
An SLCT is a rare sex cord stromal tumor, whose imaging features were seldom researched. Our study showed that SLCTs were most frequently detected in young women, with virilization as the common symptom. Besides, solid components of the tumor were isoattenuated on the conventional CT scan, and exhibited isointensity or slight hypointensity relative to adjacent myometrium on T1WI and T2WI. Additionally, notable enhancement was observed on contrast-enhanced imaging. DICER1 hotspot mutations were common in SLCTs.
SLCTs are the most common virilizing ovarian tumors, known as androblastomas or arrhenoblastomas. SLCTs usually arise in young adults, with an average age of 25 at diagnosis.3,5 Most SLCTs were unilateral, and a tiny minority were bilateral.7,8 Androgen overproduction always causes hirsutism, acne, deepening of the voice, breast atrophy, and clitorimegaly in SLCT patients. 2 In our study, patients showed irregular menstruation, hirsutism, acne, rougher voice, and breast development stagnation. Besides, serum testosterone levels were elevated in three young patients. The tumor is rarely estrogenic. 9
SLCTs could be solid, cystic, or solid-cystic masses. The solid-cystic type is most common, accounting for 60% of ovarian SLCTs, 5 which is consistent with our findings. According to the degrees of differentiation of tubules formed by Sertoli cells, SLCTs are divided into well-differentiated, moderately-differentiated, poorly-differentiated and heterologous elements. Moderately- and poorly-differentiated tumors are the most common. Heterologous components occur in nearly 20% of SLCTs, including gastric epithelium, retinal tissue, cartilage, or carcinoid tumors.7,8,10 As a part of SLCTs, Leydig cells scatter among numerous Sertoli cells, arranging into open or closed tubules. Leydig cells are derived from the original mesenchyme of the mesonephros, and can produce and secrete testosterone, 11 making SLCTs functional ovarian tumors.
The expression of Vimentin, Inhibin-a and calretinin was detected in three patients by immunohistochemical technique, indicating that both Inhibin-α and Vimentin might help the diagnosis of SLCTs. Two of our samples harbored a DICER1 hotspot mutation, which was the p. E1705K mutation. It was established by previous studies12–15 that p. D1709N was the most common mutation hotspot in SLCTs, followed by p. E1813K. Other mutations included p. E1705K, p. D1709G, p. D1709V, p. G1809R, p. D1810Y, p. E1813Q, and p. E1813D. DICER1 gene mutation detection not only is of great significance to SLCT diagnosis, but also plays an important role in DICER1 syndrome. 16 The mutation rate of DICER1 is about 90% in the DICER1-related tumors (e.g., SLCTs, cystic nephroma, multinodular goiter, and cervical embryonal rhabdomyosarcoma), and the incidence of concomitant tumor is high. 17 It is suggested that additional genetic testing should be made for family members of patients with SLCTs or other DICER1-associated tumors. 18
Imaging plays an essential part from detection to characterization of adnexal neoplasms.19,20 The tumor was usually a solid or solid-cystic soft tissue adnexal mass but rarely a cystic mass on CT images. The MRI results suggested low SI of the solid part of the tumor on T1WI and heterogeneous moderate-high SI on T2WI. Imaging manifestations disclosed the histological components of SLCTs. SLCTs were hypervascular neoplasms, which were intensely enhanced in the artery phase. It was histologically confirmed that moderate SI parts on T2WI mainly consisted of fibrous stroma, which exhibited gradual and concentric enhancement. Compared with CT, MRI provided SLCT soft tissue images with a higher resolution. The use of contrast agents also enabled the evaluation of mass vascularity and delivered much more information such as the shape, boundary, and pelvic lymph nodes. The combination of CT and MRI scanning with contrast enhancement offered us more valuable imaging information and narrowed the diagnosis range.
SLCTs should be differentiated from such ovarian neoplasms as granulosa cell tumors, fibrothecoma, and sclerosing stromal tumors. Granulosa cell tumors are usually gestrogen tumors and rarely androgenic tumors. It is reported that the adult granulosa cell tumor has a typical “sponge-like appearance.” 21 These features can facilitate the differentiation between granulosa cell tumors and SLCTs. In addition, fibrothecoma shows similar SI with SLCTs because its contains fibrous tissue, and most patients with fibrothecoma are in their postmenopausal period. 22 As for sclerosing stromal tumors, they always occur in young women and have similar features as SLCTs, which renders it difficult to distinguish sclerosing stromal tumors from SLCTs by imaging before surgery. 23
There are several limitations of this study. Firstly, this study had a sample size, with only five cases reviewed. Secondly, the data of patients were incomplete, especially for DICER1 gene testing. Lastly, the outcome of patients was missing due to the failure to follow up.
Conclusions
SLCTs are rare sex cord stromal tumors and usually diagnosed in young women. Virilization, moderate SI on T2WI and marked enhancement on contrast-enhanced imaging are indicative of SLCTs. DICER1 testing is recommended for patients with ovarian SLCTs and their family members.
Author biographies
Jingya Chen is a MD who is mainly engaged in gynecological disease research.
Yuting Liu is a MD who is mainly engaged in childhood disease research.
Yu Zhang is a MD who is mainly engaged in gynecological disease research.
Yaohui Wang is a MD who is mainly engaged in pathology research.
Xiao Chen is a PhD who is mainly engaged in gynecological disease research.
Zhongqiu Wang is a Professor who is mainly engaged in pancreatic and gynecological disease research.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Nature Science Foundation of China (grant no. 81771899), Jiangsu Province Hospital of peak academic personnel training project (grant no. y2018rc04), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (grant no. SJCX20_0514). Key Project of Chinese Medicine Technology Development Plan of Jiangsu Province (grant no. ZD201907).
Ethics approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed consent: Verbal informed consent was obtained from a legally authorized representative(s) for anonymized patient information to be published in this article.
ORCID iD: Zhongqiu Wang  https://orcid.org/0000-0002-3669-4302
https://orcid.org/0000-0002-3669-4302
References
- 1.Kommoss F, Lehr HA.Sex cord-stromal tumors of the ovary: current aspects with a focus on granulosa cell tumors, Sertoli-Leydig cell tumors, and gynandroblastomas. Pathologe 2019; 40(1): 61–72. [DOI] [PubMed] [Google Scholar]
- 2.Abu-Zaid A, Azzam A, Alghuneim LA, et al. Poorly differentiated ovarian Sertoli-Leydig cell tumor in a 16-year-old single woman: a case report and literature review. Case Rep Obstet Gynecol 2013; 2013: 858501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhat RA, Lim YK, Chia YN, et al. Sertoli-Leydig cell tumor of the ovary: analysis of a single institution database. J Obstet Gynaecol Res 2013; 39: 305–310. [DOI] [PubMed] [Google Scholar]
- 4.Gui T, Cao D, Shen K, et al. A clinicopathological analysis of 40 cases of ovarian Sertoli-Leydig cell tumors. Gynecol Oncol 2012; 127: 384–389. [DOI] [PubMed] [Google Scholar]
- 5.Young RH, Scully RE.Ovarian Sertoli-Leydig cell tumors. A clinicopathological analysis of 207 cases. Am J Surg Pathol 1985; 9: 543–569. [DOI] [PubMed] [Google Scholar]
- 6.Cai SQ, Zhao SH, Qiang JW, et al. Ovarian Sertoli-Leydig cell tumors: MRI findings and pathological correlation. J Ovarian Res 2013; 6: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez-Laguna J, Millan Y, Reymundo C, et al. Bilateral retiform Sertoli-Leydig cell tumour in a bitch. Alpha-inhibin and epithelial membrane antigen as useful tools for differential diagnosis. J Comp Pathol 2008; 139: 137–140. [DOI] [PubMed] [Google Scholar]
- 8.Rathi M, Budania SK, Khalid M, et al. Bilateral retiform variant of sertoli leydig cell tumour of ovary: an uncommon tumor with review of literature. J Midlife Health 2015; 6: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigismondi C, Gadducci A, Lorusso D, et al. Ovarian Sertoli-Leydig cell tumors. A retrospective MITO study. Gynecol Oncol 2012; 125: 673–676. [DOI] [PubMed] [Google Scholar]
- 10.Sharma S, Valiathan M, Kumar S, et al. Ovarian Sertoli-Leydig cell tumour with heterologus elements masquerading as mucinous tumour on frozen section: a case report. J Clin Diagn Res 2015; 9: ED01–ED03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makiyan Z.Studies of gonadal sex differentiation. Organogenesis 2016; 12: 42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCluggage WG, Chong AL, de Kock L, et al. Somatic tumour testing establishes that bilateral DICER1-associated ovarian Sertoli-Leydig cell tumours represent independent primary neoplasms. Histopathology 2020; 77(2): 223–230. [DOI] [PubMed] [Google Scholar]
- 13.Karnezis AN, Wang Y, Keul J, et al. DICER1 and FOXL2 mutation status correlates with clinicopathologic features in ovarian Sertoli-Leydig cell tumors. Am J Surg Pathol 2019; 43: 628–638. [DOI] [PubMed] [Google Scholar]
- 14.Schultz KAP, Harris AK, Finch M, et al. DICER1-related Sertoli-Leydig cell tumor and gynandroblastoma: clinical and genetic findings from the International Ovarian and Testicular Stromal Tumor Registry. Gynecol Oncol 2017; 147: 521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Kock L, Terzic T, McCluggage WG, et al. DICER1 mutations are consistently present in moderately and poorly differentiated Sertoli-Leydig cell tumors. Am J Surg Pathol 2017; 41: 1178–1187. [DOI] [PubMed] [Google Scholar]
- 16.Robertson JC, Jorcyk CL, Oxford JT.DICER1 syndrome: DICER1 mutations in rare cancers. Cancers (Basel) 2018; 10(5): 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz KAP, Williams GM, Kamihara J, et al. DICER1 and associated conditions: identification of at-risk individuals and recommended surveillance strategies. Clin Cancer Res 2018; 24: 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schultz KAP, Stewart DR, Kamihara J, et al. DICER1 Tumor predisposition. In: Adam MP, Ardinger HH, Pagon RA, et al. (eds) GeneReviews ® [Internet]. 2014 Apr 24 [Updated 2020 Apr 30]. Seattle (WA): University of Washington, Seattle, 1993–2021. [Google Scholar]
- 19.Kennedy A, Woodward P.Imaging in gynecology. Top Magn Reson Imaging 2010; 21: 199. [DOI] [PubMed] [Google Scholar]
- 20.Perera DS, Prabhakar HB.Imaging of the adnexal mass. Clin Obstet Gynecol 2015; 58: 28–46. [DOI] [PubMed] [Google Scholar]
- 21.Kim SH, Kim SH.Granulosa cell tumor of the ovary: common findings and unusual appearances on CT and MR. J Comput Assist Tomogr 2002; 26: 756–761. [DOI] [PubMed] [Google Scholar]
- 22.Schultz KA, Harris AK, Schneider DT, et al. Ovarian sex cord-stromal tumors. J Oncol Pract 2016; 12: 940–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atram M, Anshu Sharma S, et al. Sclerosing stromal tumor of the ovary. Obstet Gynecol Sci 2014; 57: 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]