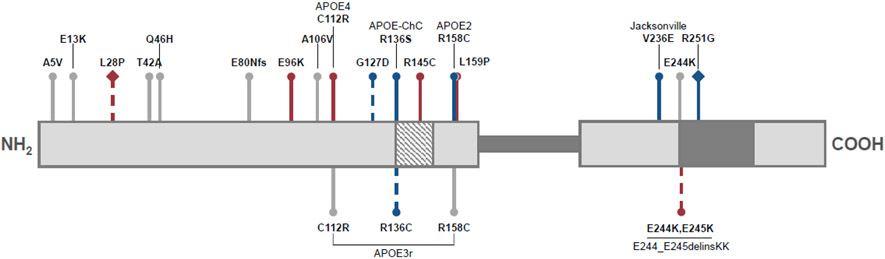
Figure 1. Numerous nonsynonymous APOE mutations modify neurological disease risk.
The mature APOE protein consists of two primarily alpha-helical domains (light grey sections), connected by an intrinsically disordered hinge region (dark grey bar; residues 200-215). The N-terminal domain contains the low-density lipoprotein receptor binding site (diagonal stripes; residues 136-150) while the C-terminal domain contains sites for lipid binding (dark grey; residues 244-272). In relation to APOE3 (C112; R158), risk mutations are depicted in red, protective mutations in blue, and mutations of unknown neurological effect are in grey. Dashed lines indicate predicted or mild directional effects. Diamond-headed pins reflect variants on an APOE4 (C112R) background. This diagram was adapted from data in the Alzforum APOE mutations database. Abbreviations: fs represents a frameshift mutation, ChC stands for Christchurch, delins is a deletion-insertion mutation.