Fig. 1.
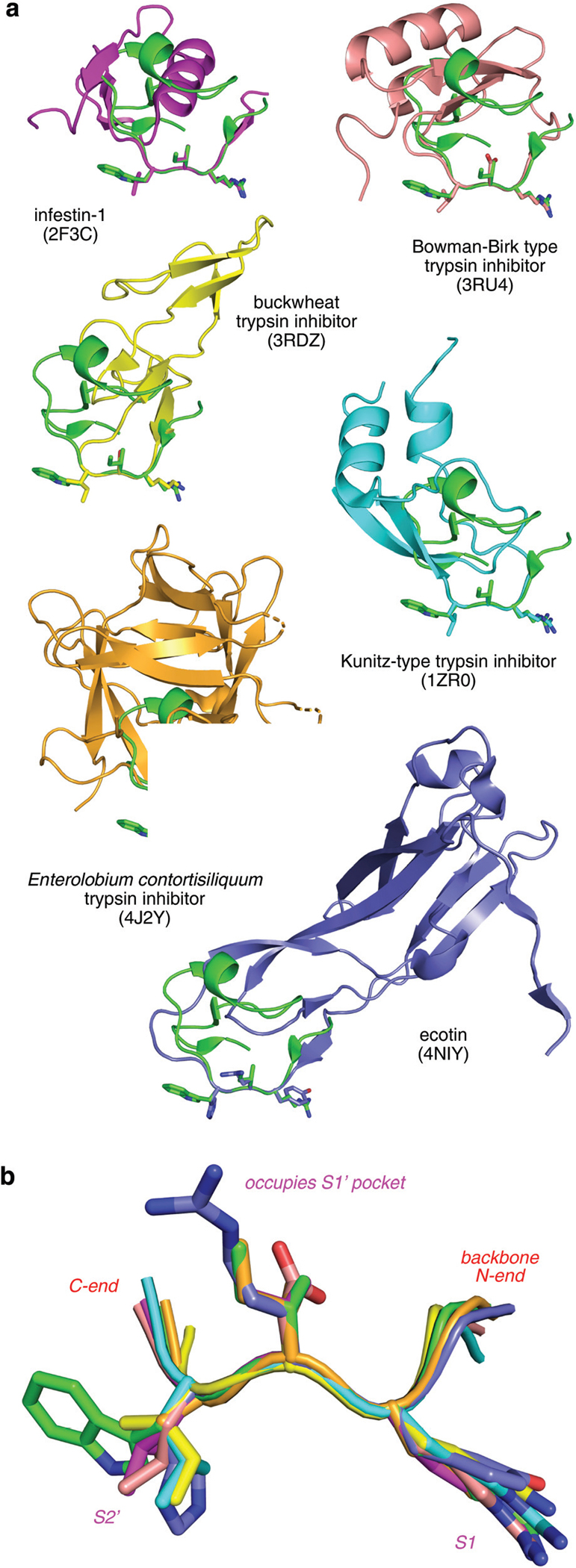
Overlay of the interface regions of natural trypsin inhibitors showing similarities in the trypsin-binding segment, ie the highly conserved PPI interface. (a) Momordica charantia trypsin inhibitor A (MCTI-A, green, PDB ID 1F2S) is overlaid on six other trypsin inhibitors. (b) Enlarged view of the overlay with side chains of the P1, P1’, P2’ residues are highlighted as sticks.
