Abstract
Optimism bias is the tendency to believe desirable events are more likely to happen than undesirable ones. People often display optimistic biases for themselves (personal optimism), but also for members of groups they like or identify with (social optimism). However, the neural bases of and connections between these two concepts are poorly understood. The present study hence used both questionnaires and a social optimism task performed during magnetic resonance imaging to investigate how network connectivity associates with personal and social optimism biases. Using sparse canonical correlation analysis, we found that a behavioral dimension that included both in‐group optimism bias and personal optimism bias was positively associated with a dimension of network connectivity. This dimension comprised two networks with positive weights (dorsal precuneus‐related default mode network and dorsal sensorimotor network), and three with negative weights (including parts of the salience and central executive networks). Our findings indicate that connectivity in networks adjacent to the temporoparietal junction favors propagation of both personal and social optimism biases. Meanwhile, low connectivity in more frontal networks associated with more complex cognition may also further such propagation.
Keywords: functional connectivity, optimism bias, social optimism
The present study hence both questionnaires and a social optimism task performed during magnetic resonance imaging to investigate how network connectivity associates with personal and social optimism biases. The study found that a behavioral dimension that included both in‐group optimism bias and personal optimism bias was positively associated with a dimension of network connectivity. Our findings indicate that connectivity in networks adjacent to the temporoparietal junction favors propagation of both personal and social optimism biases.

1. INTRODUCTION
Optimism bias refers to the belief that desirable events are more likely to happen in the future than undesirable events. The majority of the general population are prone to a personal optimism bias, that is, seeing their own future in overly bright colors (Windschitl & Stuart, 2015). Such positively biased expectancies may be highly beneficial in that they promote mental and physical well‐being (Conversano et al., 2010; Rasmussen et al., 2009). Additionally, recent literature suggests that there also exists a social optimism bias (Aue, Dricu, Moser, et al., 2021; Dricu et al., 2018). Specifically, people display an optimism bias for members of groups they identify with (in‐groups) or like. At the same time, they may show a pessimism bias for members of out‐groups (groups they do not identify with) or that they dislike (especially if these are perceived as cold and incompetent). Both social phenomena may be interdependent, because imagining something bad happening to the ones we do not like may (de)activate the same brain regions as imagining something good happening to the ones we like (Aue, 2014).
The literature on the neural foundations of social optimism biases, focusing on brain structure, brain activation and functional connectivity, is limited. One study (Moser et al., 2021) investigated how personal and social optimism biases relate to brain functional connectivity during resting state. This study revealed a link between social optimism bias and personal pessimism with connectivity in a variety of networks including the ventral DMN (which comprised the middle temporal gyrus (MTG), parts of the insula, IFG, and hippocampus), but also the anterior DMN (which comprised the dorsal anterior cingulate cortex (ACC)), dorsal precuneus DMN, as well as dorsal sensorimotor network and lateral fronto‐parietal CEN.
Relatedly, the examination of the association between task‐accompanying brain activity and social optimism biases (A. J. C. Cuddy et al., 2007; Dricu, Schupbach, et al., 2020; SCM, Fiske, 2015; Fiske, 2017) used the Stereotype Content Model (SCM, A. J. C. Cuddy et al., 2007; Fiske, 2015). SCM divides social groups along two dimensions of perception: warmth and competence. While warmth refers to the attribution of interpersonal intentions and capabilities to a social target, competence relates primarily to what personal intentions and faculties are attributed. Different placement among the four quadrants of these two dimensions may result in experiences among observers that qualitatively differ in emotionality, attitudes, and subsequent behavior (Aue, Buhrer, Mayer, & Dricu, 2021; A. J. C. Cuddy et al., 2007; Fiske, 2015, 2017). The study on social optimism bias and task related brain activation (Dricu, Schupbach, et al., 2020) identified medial regions of the DMN, namely the posterior cingulate/precuneus and ventromedial prefrontal cortex to be more strongly activated during the evaluation of the likelihood of events happening to an in‐group vs. out‐groups on the different quadrants of the SCM (three different out‐groups in this specific case: (1) out‐group elderly individual, perceived as high in warmth and low in competence; (2) out‐group businessperson, perceived as low in warmth and high in competence; (3) out‐group alcoholic individual, perceived as low in both warmth and competence). Importantly the strength of the optimism bias revealed for the in‐group varied as a positive function of the strength of said brain activation. Conversely, the IFG and the temporo‐parietal junction (TPJ) were more strongly activated during likelihood ratings for cold‐out‐groups than for the in‐group; these regions' activations, too, were positively correlated with optimism biases—but this time for the cold out‐groups. Still another study (Aue et al., 2012) investigated event‐related neural activity and connectivity associated with social optimism bias. In that study, American football fans predicted the likelihood that their favorite (presumed in‐group) or least favorite (presumed out‐group) team would win a game. Consistent with expectations participants displayed a social optimism bias in that the favorite team trials were characterized by higher attributed winning odds than were the least favorite team trials. Furthermore, activity in a cluster comprising parts of the left inferior occipital and fusiform gyri distinguished between favorite and least favorite team trials. More importantly, functional connectivity of this cluster within the visual network (VIS) with the human reward system was specifically involved in the optimism bias expressed in behavior. These findings hence support the idea of a visual attention bias generating or contributing to the optimism bias.
Measures of brain structure may elucidate more stable patterns of association with biases, underlying brain function and connectivity. Brain structure (Moser et al., 2020) was linked to an optimism‐pessimism dimension including reduced optimism/increased pessimism biases for out‐groups perceived as cold (e.g., businesspersons and alcoholics). Specifically, this optimism‐pessimism dimension was associated with cortical thickness in the left insula, but also in the inferior frontal gyrus (IFG) and several other regions that are part of either the default mode network (DMN) which is active during rest and involved in self‐referential processing, or the central executive network (CEN) important in planning. Further, the anterior insula and IFG have been linked to the way people update beliefs relevant to personally relevant optimism biases in the face of new information (Kuzmanovic et al., 2016; Moutsiana et al., 2015). Within this optimism‐pessimism dimension a bias towards characters perceived as warm—warmth itself is linked to trust (Oyediran et al., 2018; Shkurko, 2013)—played an important role.
In sum, the existing literature links social optimism biases with networks that are involved in general processing, but also networks implicated in the evaluation of the valence of stimuli or the differentiation of self and others (Dricu, Schupbach, et al., 2020; Moser et al., 2020; Moser et al., 2021). However, to our knowledge, no study has yet investigated the association of task‐related neural connectivity with optimistic and pessimistic biases for an in‐group and more than a single out‐group. In addition, social optimism bias has not been compared with personal optimism bias in this respect.
The present study aimed to remedy this lack of research by examining the link of local brain connectivity with optimism/pessimism (bias) both on a personal level (i.e., assessed through questionnaires), as well as on a social level (i.e., assessed with an experimental task). In the experimental task (previously validated in a student population; Dricu et al., 2018), our participants (who were students) rated the likelihood of future events for four kinds of characters while in a magnetic resonance imaging (MRI) scanner. The four characters referred to four different groups on the four different quadrants of the SCM—an in‐group (students) which was perceived as warm and competent, and three qualitatively different out‐groups: a group perceived as warm but not competent (elderly), one perceived as competent but not warm (businesspersons) and another one perceived as neither warm nor competent (alcoholics). Additionally, participants identified most strongly with the in‐group characters as indicated by the “other in the self” scale (Aron et al., 1992; Aue, Buhrer, Mayer, & Dricu, 2021). Using these methods the present study will allow to see whether resting‐state related findings concerning optimism networks can be extended to task‐related neural activity. Moreover, the study will allow to assess the neural networks of general optimism and related measures and compare it with the networks of social optimism biases (i.e., assess their overlap). This is important as overlapping results would speak to the brain possessing an internal baseline for the neural manifestation of a large array of different optimistic phenomena, ranging from trait optimism over personal optimism bias to social optimism bias.
We hypothesized (H1) that a behavioral dimension of personal and in‐group related social optimism, on the one hand, and personal pessimism and optimism for cold out‐groups, on the other hand, would be associated with the average connectivity during this task within 12 task‐related networks (Dricu, Schupbach, et al., 2020; Moser et al., 2020; Moser et al., 2021). Considering the network connectivity, we postulated (H2) that associations would focus on regions that have previously been linked with optimism biases in brain imaging literature such as the IFG, insula, TPJ, ACC, and related network partitions of the salience network, medial core regions of the DMN and CEN networks (mostly lateral fronto‐parietal subnetworks).
2. METHODS
2.1. Participants
Recruitment was undertaken via multiple avenues, including the university's local participant pool, e‐mails, as well as flyers. To be included, participants had to be students capable of undergoing an MRI scan. Forty‐nine healthy men and women participated. Further information and results on task activation and brain structure can be found elsewhere (Dricu, Schupbach, et al., 2020; Moser et al., 2020). One participant was excluded for failure to complete the questionnaires (which are detailed in the Procedure section). An additional participant was excluded because more than 60% of the participant's connectivity values were outliers (more than 3 standard deviations away from the group mean). Another participant's data was removed because all but one session were characterized by excessive motion artifact (>2 mm motion). Finally, one more participant was excluded because of extreme behavioral ratings given during the task (i.e., frequently assigning the values “0%,” “50%,” or “100%,” and doing so more than three standard deviations more often than the rest of the sample). The final sample used for the present study comprised 45 participants (32 women, 13 men; mean age = 23.2 years, SD = 4.2 years, range 19–36 years).
2.2. Procedure
All participants gave informed consent prior to participation and data has been anonymized. The procedures and consent form had previously been approved by the ethics review board of the canton Bern and are in accordance with the declaration of Helsinki. Prior to scanning, participants completed several questionnaires through use of an online portal. Apart from a sociodemographic questionnaire, the present study included the German versions of: (a) the Comparative Optimism Scale (COS; measuring self‐related future expectancies as compared with those for others; Weinstein, 1980), (b) the Revised Life Orientation Test (LOT‐R, measuring trait optimism; Glaesmer et al., 2008; Scheier et al., 1994), and (c) the Rosenberg Self‐Esteem Scale (RSES, assessing personal self‐esteem; Rosenberg, 1965; Von Collani & Herzberg., 2003).
Optimism bias task: For 16 desirable and 16 undesirable validated events, each participant rated how likely the event was to happen to each of the four fictional characters. The validation of the paradigm (Dricu et al., 2018) had assessed these events in terms of both perceived controllability and perceived frequency with respect to the general population and ensured that they were matched across the two desirability conditions. We further assessed the characters on the dimensions of their perceived warmth and competence. In addition, we checked the participant's identification with the characters using the “Inclusion of Others in the Self” (IOS) scale (Aron et al., 1992). Trials began with a fixation cross of random duration (1.5–3 s), followed by a screen containing the target event. The 32 events displayed an animated version of the character and the given scenario in the middle of the screen, while a short description of the target event together with a visual analog rating scale ranging from 0% to 100% were presented at the bottom (see Figure 1). Trials were displayed in a pseudorandomized order (Latin square). For purposes of MRI acquisition, the trials were divided into four runs of 7 min each.
FIGURE 1.
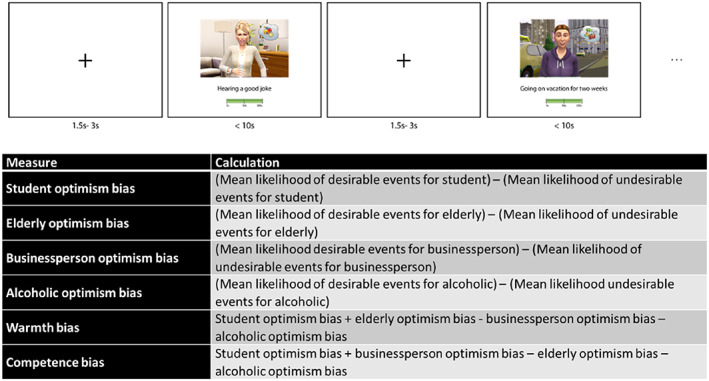
Summary of the task. Top: Visual depiction of a trial, bottom: description of the formulas for the six task‐related measures.
The “Sims 4” was used to produce backgrounds, characters, and scenarios. Stimuli were matched in brightness and contrast. Female participants saw female animated characters and male participants viewed male animated characters. As previously validated (Dricu et al., 2018), each character represented a social group that—within the Stereotype Content Model's two‐dimensional space—mapped onto a specific quadrant of high or low warmth and competence (A. J. Cuddy et al., 2011; Fiske, 2015). Validation had indicated that students and businesspeople are perceived as overall more competent than are elderly and alcoholics. Because the present sample consisted of students, the student character was considered an implicit in‐group character (both high in warmth and competence). The other characters were considered out‐groups located on different quadrants: high warmth/low competence being represented by an elderly person, high competence/low warmth by a businessperson, and low warmth/low competence by an alcoholic person. Characters were carefully cropped on 32 backgrounds pertinent to the target events.
Experiment data were initially cleaned from participants giving consistently extreme values and generally from likely non‐answers as described elsewhere (Moser et al., 2020). For every remaining participant, we then computed six task measures (1–6, see Figure 1). Specifically, an optimism bias was calculated for each character (1–4). This was done by first z‐standardizing all likelihood estimate values within each participant. Next, we subtracted the likelihood means of undesirable from those of the desirable events for each character. Additionally, we created a warmth bias (5) as the mean optimism biases for the student and elderly characters minus the mean optimism biases for the businessperson and alcoholic characters. Similarly, we computed a competence bias (6) by subtracting the mean optimism biases for the characters estimated less competent (elderly and alcoholic) from the optimism biases of the characters estimated more competent (student and business person, Dricu et al., 2018).
2.3. MRI data acquisition and preprocessing
All MRI images were acquired using a 3 Tesla Siemens Magnetom Prisma Scanner (Siemens, Erlangen, Germany) with a 64‐channel head coil at the Insel University Hospital in Bern, Switzerland. Volumes were registered using a T2*‐weighted multiband echo‐planar imaging sequence (multiband EPI) with 48 slices covering the whole brain (TR/TE = 1000 ms/30 ms; 0.5 mm gap; interleaved slice order; flip angle = 80°; field of view = 192 × 192 mm; matrix size = 96 × 96; voxel size = 2 × 2 × 2.5 mm; PAT mode GRAPPA; acceleration factor = 2; multiband factor = 3). An anatomical scan (MP‐RAGE; 1 mm isotropic voxels; TR = 2300 ms; TE = 2.98 ms; flip angle = 9°; matrix size = 256 × 256) was conducted before the functional runs to get highly resolved structural information for the normalization procedure. Functional scans were divided into four runs.
The MATLAB R2017 (MathWorks) based toolboxes DPABI (Yan et al., 2016) and SPM12 (www.fil.ion.ucl.ac.uk/spm/software/spm12/) were used for preprocessing. All DICOM images were converted to NIfTI format and the initial and final five volumes were removed. The remaining fMRI data were motion corrected to the first volume via rigid‐body alignment; further, cross modality coregistration was performed between the anatomical T1 scan and functional scans; followed by spatial normalization of the functional images into Montreal Neurological Institute stereotaxic standard space and smoothing with a 4 mm full width at half‐maximum Gaussian kernel. Additionally, data were detrended, underwent multiple regression with motion parameters and their derivatives (24‐parameter model) (Friston et al., 1996), and we also applied the CompCor noise reduction method (five principal components) (Behzadi et al., 2007), taking into account white matter (WM), cerebrospinal fluid (CSF) time series, and their linear trends.
2.4. Independent component analysis and functional connectivity
Prior to connectivity analysis we performed group independent component analysis (ICA) using the Group ICA of fMRI Toolbox (GIFT, https://trendscenter.org/software/, version 3.0c). We chose to conduct ICA— as opposed to use predefined networks—because of the high likelihood of the experimental task having its own composition of networks, which may be different from other tasks or resting state. Using Infomax algorithm (Bell & Sejnowski, 1995), we extracted 20 spatially independent components (ICs) across all participants, including the three runs with the least motion for each participant (Calhoun et al., 2001; Calhoun et al., 2009). Applying ICASSO, the analysis was repeated 20 times for assessing the repeatability (Himberg et al., 2004). The 20 ICs were evaluated to identify functionally relevant brain networks. The criteria for retaining networks were: (1) the peak clusters of a network should be in the grey matter, and (2) keeping the overlap with known brain edge areas as well as vascular, ventricular and artifact susceptible regions to a minimum. Based on these criteria 12 networks were selected for further analysis (Figure 2). For simplicity of understanding, we assigned names to these networks that are—whenever possible—in alignment with the general literature and indicate whether they are likely to be part of the default mode network (DMN), central executive network (CEN), salience network, visual network (VIS), or sensorimotor network (SMN, Doucet et al., 2019; Smith et al., 2009). One network (network 10, IFG‐Insula‐MTG) did not easily fall within these, but contained numerous regions (in particular IFG and insula; regarding both brain structure and function) that are associated with optimism, optimism bias, and optimistic belief updating (Dricu, Kress, & Aue, 2020; Moser et al., 2020; Moutsiana et al., 2015). That same IFG‐Insula‐MTG network also contained significant parts of the medial temporal gyrus (MTG) that lead (from an inferior direction) to the TPJ, as well as precentral regions. Notably, this network strongly overlaps with the medial temporal DMN of a prior paper (Moser et al., 2021) when it comes to MTG and parts of the IFG, but not subcortical and precentral regions. Lastly, we also identified a network (referred to as the salience‐medial DMN) that combined parts of salience network designations (posterior ACC and anterior insula, parts just superior of the right TPJ, plus parts of the lateral prefrontal cortex, but no other subcortical structures) with medial parts of the DMN (both more anterior and posterior cingulate cortices, and precuneus).
FIGURE 2.
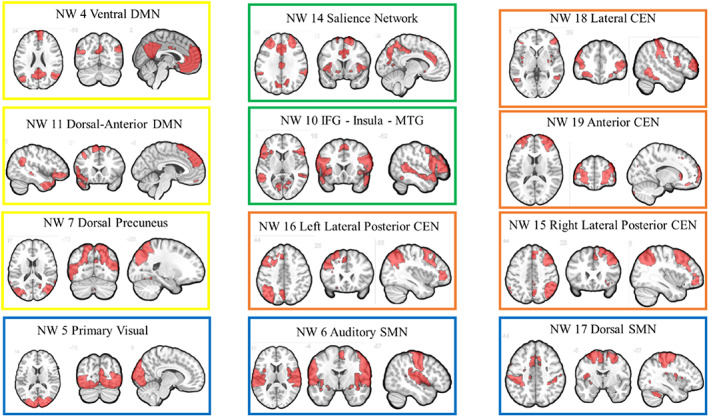
Spatial maps of the 12 networks identified across fMRI sessions and participants. DMN, default mode network; ECN, executive control network; IFG, inferior frontal gyrus; MTG, medial temporal gyrus; NW, network; SMN, sensorimotor network.
Next, we extracted the time courses of neural activity within each of those 12 networks for each participant and session. Prior to calculation of the functional network connectivity (FNC) measures, data were detrended and temporally filtered (0.01–0.15 Hz) across all participants and sessions, using the GIFT toolbox' default options. We then computed session‐specific connectivity and averaged participants' connectivity across the three sessions with the least motion. (integration) FC. Within‐FNC‐connectivity (WFC) was computed as the average correlation of all voxel within the network with each other. Between‐FNC‐connectivity was computed as the correlation between the average time series of each of FNCs with each of the other retained FNCs average time series. Additionally, we calculated the average volume‐to‐volume head motion for each participant and regressed it out from our results to ensure no association between head motion and the behavioral optimism variate (see next section for a detailed description). This did not severely alter connectivity results, as all network connectivities correlated at r > .91 before and after this step.
2.4.1. (Sparse) canonical correlation analysis
In order to associate the the network connectivity and the behavioral optimism data set, we used a sparse canonical correlation analysis (sCCA) algorithm (Witten et al., 2009), in order to verify whether one data set associates with the other in a broadly similar manner as previously described (Moser et al., 2020; Moser et al., 2021). sCCA and similar methods have become more and more commonly used in the domain of neuroimaging (Zhuang et al., 2020). In short, sCCA specifies pairs of canonical variates by applying weights to variables in Data set 1 (non‐imaging data) and variables in Data set 2 (connectivity data) that best express the maximal correlation (i.e., canonical correlation) between the two data sets. The correlations between the canonical variates are the canonical correlations. The reason for our selection of this method was that—unlike multiple regression for example—it allows the association of an entire dataset with another entire dataset and not only one potential outcome variable. Our sCCA approach used an L1 penalty function.
For the non‐imaging optimism dataset, we used 11 variables: the six aforementioned task measures (optimism bias for each target character, warmth bias, and competence bias), two subscales each for the LOT‐R and COS (optimism and pessimism subscales for each of them), and the RSES overall score, given its close relationship with optimism (Scheier et al., 1994).
Prior to conducting the sCCA, all variables were z‐standardized, and remaining outliers (>3 standard deviations away from the mean) were replaced with mean values. If this created new outlier values these too were replaced with mean values. We tested the first six modes for significance and reliability, because—depending on assessment of explained variability—they explained more than 90% of variability in our data. Apart from an analysis for within‐network connectivity, we also performed a sCCA with a connectivity dataset with 66 between‐network connectivities (i.e., all measures of connectivity between the 12 networks).
2.4.2. Reliability analysis
We performed the following reliability analyses:
We retested the analysis without putting outliers to mean values and instead converting each variable to ranks prior to performing sCCA.
(a) Cross validation using training and test sets. We randomly resampled half the sample 10,000 times to use them as training sets, performed an sCCA on each of these training sets, and then applied the identified weights from each training set on the test sets (the other half of the sample). (b) Mean and standard deviation of Moser's redundancy‐reliability score (RR‐score). Moser's RR‐score is used to measure the stability of the variable‐to‐variate correlations. It investigates whether different subsets of the data would produce similar associations between variables and variates (Moser, 2018; Moser et al., 2018).
2.4.3. Post hoc analyses
We also provide (univariate) Spearman correlations between all variables of both datasets, including correlations of these variables with the sCCA variates of the first six modes. The results can be found as part of Supporting Information.
3. RESULTS
We found that the second (r = .67, p = .006), fourth (r = .58, p = .27), and fifth (r = .58, p = .009) modes were significant in the within FNC sCCA. Given the results of subsequent reliability analyses (see below), we will only present and discuss weights of mode 2 in the manuscript. Modes 4 and 5 as well as weights of all other modes are part of Supporting Information (Table S1, Figures S1 and S2).
The highest positive weights concerning the optimism variate were assigned to the student optimism bias (0.83), the COS optimism subscale (0.36), and the LOT optimism subscale (0.29), while the algorithm assigned the highest negative weight to the LOT pessimism subscale (−0.20, see also Figure 3, and Table S1A).
FIGURE 3.
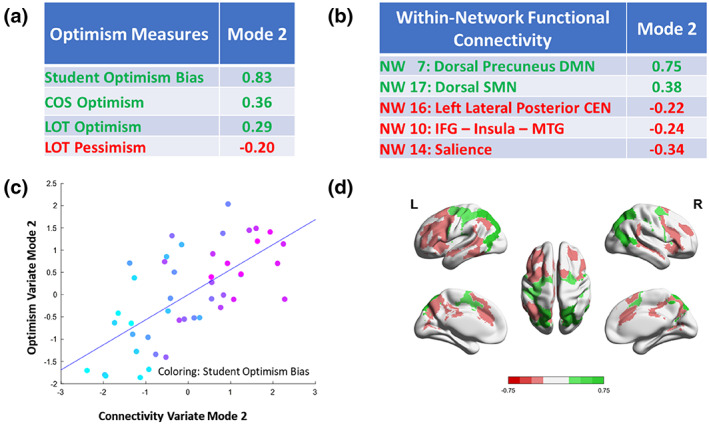
Depiction of results for mode 2 of the sCCA. Panel (a): Weights greater than 0.2/lower than −0.2 for the optimism variate. (b) Weights greater than 0.2/lower than −0.2 for the network connectivity variate. (c) Scatter plot of the two variates. (d) Visual depiction of the networks and their weights. CEN, central executive network; COS, comparative optimism scale; DMN, default mode network; IFG, inferior frontal gyrus; LOT, life orientation test; MTG, medial temporal gyrus; NW, network; SMN, sensorimotor network.
The algorithm attributed the highest positive weights concerning the network connectivity variate to the dorsal precuneus part of the DMN (0.75) and the dorsal subnetwork of the SMN (0.38); the most pronounced negative weights were assigned to the anterior salience network (−0.34), the network containing the IFG and insula (−0.24), and the left lateral posterior CEN (−0.22, see also Figure 3, and Table S1B).
3.1. Reliability analyses
After conducting the reliability analyses, we found that—among the significant results—only mode 2 appeared stable. Mode 2 was also more reliable than the other significant modes. Specifically, mode 2 had positive correlations across the majority of cross validations (mean r: mode 2 = 0.15, mode 4 = 0.00, mode 5 = −0.02), and also had a slightly higher Moser's RR‐score, which—as could be expected—had one peak and appeared skewed towards the right (high values; mean RR scores (SD): mode 2 = 0.60 (0.19); mode 4 = 0.49 (0.20); mode 5 = 0.52 (0.20); see also Figure S3). Modes 2 and 4 (but not 5) were also significant in the alternative analyses that included raw variables (i.e., not converted into ranks; mode 2: r = .66, p = .008; mode 4: r = .67, p < .001; mode 5: r = .48, p = .14). With respect to stability, the weights of mode 2 correlated highly with those of the alternative analysis, while this was not the case for modes 4 and 5 (mode 2: r = .98; mode 4: r = .03; mode 5: r = −.02). Low correlations were likely due to modes having switched positions, that is, weights of new mode 4 correlated highly (r = .88) with those of initial mode 5 and vice versa (r = .96), wherefore mode 5 in the original analysis should be considered significant and mode 4 as nonsignificant in the secondary analysis.
Between‐FNC sCCA did not find any significant results. This was not overly surprising given the increased need of statistical power with the higher number of variables and the reduced power compared to our previous study (Moser et al., 2021). Seeing as this would render interpretation difficult, we are not going to discuss said results.
4. DISCUSSION
The present study investigated within‐network connectivity related to personal and social forms of optimism and pessimism. In accordance with our predictions, we found that connectivity within networks was associated with a dimension that ranged from optimism bias expressed for an in‐group, on one side, to trait pessimism (LOT pessimism), on the other. This dimension, therefore, is fairly similar to a behavioral optimism dimension we identified with a different social optimism task and resting‐state connectivity (Moser et al., 2021). This earlier resting‐state study had further revealed another significant (but less reliable) mode that included the questionnaire (COS and LOT) optimism measures, both of which are part of our significant mode 2 finding here, and it appears reasonable to assume that mode 2 in the current study is a combination of both of those modes found in the resting‐state study.
The assumption of similar results for task‐accompanying and resting‐state connectivity is further supported by closer inspection of the networks involved in the findings of the present study, which overlap with the ones found to be most important in the resting‐state study (Moser et al., 2021). In this previous study, there was a first relevant mode including social optimism during resting state, which revealed uniformly negative behavior‐local connectivity associations (i.e., more social optimism was linked with less within‐network resting‐state FNC). The second significant mode of the resting‐state study showed a highly similar pattern to mode 2 of the present study. In both cases, a dimension including comparative and self‐centered trait optimism, was positively associated with connectivity within networks of the dorsal precuneus DMN and dorsal SMN, while connectivity within the salience and lateral fronto‐parietal CEN networks were negatively associated.
Low within‐network functional connectivity could be interpreted as a sign of low functional integration, that is, a brain where networks are less focused on their specific functions that are exerted by between‐regions interactions within the network. Instead, these networks potentially underlie greater influences by other networks. We have previously hypothesized that low modularity—particularly in brain areas associated with higher order cognition—would be beneficial to the propagation of social biases (Moser et al., 2021).
Among the networks whose connectivity contributed negatively to the optimism variate are two networks (left lateral posterior CEN and salience network) that comprise regions that border the TPJ but also include parts of the prefrontal cortex. The observed negative weights for these two networks that combine regions to which the literature assigns different (but not necessarily independent) subfunctions, possibly signals reduced likelihood of personal forms of optimism/pessimism to go hand in hand with social optimism biases when these networks are in lockstep. Given the proposed importance of the TPJ for the differentiation between self and others (Knyazev et al., 2021; Quesque & Brass, 2019; Speitel et al., 2019; Steinbeis, 2016), a reduced distinction between self and other (here likely indexed by reduced local connectivity within the two networks) may be responsible for pronounced social biases to arise. We further speculate that the less control the left lateral posterior CEN exerts during the task (likely indexed by reduced local connectivity in the current study), the lower the personal and social optimism biases displayed.
The cortical salience network and its parts have been indicated to be important to the regulation of the stimuli's importance (Ligeza et al., 2016) and working memory (Owen et al., 2005). it is noteworthy however, that what we termed salience network here, also includes not only subcortical regions associated to salience, but also regions that in the resting‐state study were part of the medial DMN. Some of the anterior midline structures have been shown to be important to stimulus value evaluation in terms of valence (Monosov, 2017; Viinikainen et al., 2010). The limited connectivity within the present salience network may allow for less encapsulated and specific processing, allowing for a more efficient propagation of biases arising at earlier stages and less clear differentiation of networks from each other. Hence, the finding in mode 2 may indicate that, when connectivity within the salience network is low, the personal optimism more easily extends into social optimism bias.
One of our findings concerned the network including the IFG, insula, and MTG. This network thus comprises important key areas identified by research on optimism and related concepts (Dricu, Kress, & Aue, 2020). Given the importance of the network's regions to autobiographic memory and facial stimulus integration (Herlin et al., 2021) as well as to evaluation of stimuli to personal well‐being and pain (Brooks & Tracey, 2007; Segerdahl et al., 2015), that network may be particularly well suited for the complex task of associating personal preferences and needs within the general experience of the individual. One could speculate, that failure to do so in a highly synchronized way (as indicated by reduced within network connectivity), may allow for extension of biases towards others (i.e., social optimism biases). In the present study, this network's local connectivity was inversely related to the degree of personal and social optimism displayed. In the earlier resting‐state study, the network most similar to the current IFG‐insula‐MTG network was the ventral DMN subnetwork including the MTL. Interestingly, in this prior study, increased resting‐state within‐network connectivity in the medial temporal DMN was associated with reduced social optimism but increased personal optimism (Moser et al., 2021). Hence, the difference in weightings for the current and prior findings may point to the role of said network differing between at rest vs. during tasks in regard to the personal component of optimism. The negative weight assigned to the IFG‐insula‐MTG network here, may therefore point to the networks impact on decision making if a personal optimism is extended to other members as social optimism bias, which requires further investigation. The fact that personal bias appears inversely associated with connectivity in these similar networks in both studies, may point to the possibility that the directionality of the association with measures of personal optimism is more variable.
Notably, only two networks (dorsal precuneus DMN and dorsal SMN) were characterized by positive weights on the neural mode 2 variate. Post hoc results indicated that mode 2 was substantially carried by a positive correlation between connectivity within the dorsal precuneus DMN and the student optimism bias, which is a proxy for social optimism for the in‐group. Activity in the dorsal precuneus has been reported to be involved in social optimism bias before (Aue et al., 2012). Moreover, in a study on spider phobia, the same region was characterized by markedly reduced activity during situational inadequate catastrophizing expectancies (Aue et al., 2015). Hence, whereas increased activity and connectivity within the precuneus appear to foster (social) optimism bias, its deactivation and reduced local connectivity may go hand in hand with reduced mental health indicators. The precuneus is an important integration center in the brain, connecting information from various domains (see Cavanna & Trimble, 2006, for an overview of its potential functions), subserving self‐regulation. Among others, the precuneus has been shown to be involved in the adoption of a third‐person perspective (Vogeley et al., 2004), as well as reflection of one's own and others' feeling states (Ochsner et al., 2004), which all should be crucial for personal and social (in‐group) biases to arise. This structure has further been revealed to be implicated in top‐down driven selective attention (Hahn et al., 2006). Our current observations are thus in line with prior findings (Aue, Dricu, Singh, et al., 2021; Kress et al., 2018; Kress & Aue, 2019; Singh et al., 2020) suggesting the involvement of selective attention in optimistic biases and related concepts (for a theoretical overview, see Kress & Aue, 2017).
Like the precuneus, the dorsal SMN connectivity also contributed positively toward the mode 2 behavioral (social) optimism variate. The sensorimotor cortex can be considered as part of the extended mirror‐neuron system (Pineda, 2008) and is essential for emotional communication and regulation (Williams et al., 2020). It thus is not surprising that this network appears in the context of favorable social biases (here: in‐group social optimism bias). Self‐involvement as a consequence of sensorimotor perspective taking for the in‐group may trigger the same kind of regulative actions as direct self‐relevance. Both biases may therefore initiate comparable actions that result in the positive treatment of the self and the in‐group (expressed by personal and social optimism bias in the current study).
Finally, our data indicate that, in terms of brain connectivity, optimism measured within a social optimism bias task and personal optimism as assessed by questionnaires fall upon the same dimension. It thus is likely that both forms of optimism rely on at least partially overlapping neural functioning in the identified networks. Whereas the propagation of optimistic biases may be assumed to be overall advantageous, as high optimism is generally associated with enhanced health (Conversano et al., 2010; Rasmussen et al., 2009), it is necessary to acknowledge, that the extension of personal optimism to others cannot be easily interpreted as a positive or negative thing. On the one hand, being optimistic about in‐groups reduces anxiety about the future of people and groups one is invested in or cares about. Such a reduction in anxiety should then ultimately be expressed by increased well‐being. On the other hand, social optimism/pessimism bias is based on stereotypical processing and goes along with preferential treatment of some people (e.g., members of in‐group and warm out‐groups) over others (e.g., members of cold and incompetent out‐groups; Dricu et al., 2018; Moser et al., 2020), thereby penalizing a considerable number of others.
5. LIMITATIONS
While the present study underwent various types of reliability analysis, it remains possible that some currently nonreliable modes would become reliable with more participants or, alternatively, some of the currently reliable ones would turn out to not be reliable at larger sample sizes. To avoid such problems future studies may increase sample size. Still, because our current findings are highly overlapping with earlier results of a resting‐state investigation of personal and social optimism that even relied on a different experimental task (Moser et al., 2021), we are confident that our data and their interpretation are trustworthy. It is a limitation of comparison with the prior resting state study (Moser et al., 2021), that the networks employed are not exactly the same. While we named networks similarly to facilitate orientation, it is important for the reader to remember that they are not the exact same networks as in that prior study due to the networks used here being based on the social‐optimism related task. The present study analyses a general pattern of optimism and associates it with the behavioral outcome of differing conditions that happened during that task. The conditions are given even importance in the present study, in the sense that they all contribute an equal number of acquisitions to the overall analysis. However, this analysis in the present study did not look at specific connectivity characteristics for each specific condition separately. It can therefore not pinpoint which connectivities happened during said conditions, but rather points to which conditions are related to overall connectivity across the ensemble of conditions tested in the task.
We matched the gender of the shown characters to the participant's gender. It is possible that the presented gender changes some of the participant's responses. Future studies may investigate this point. Further, the present study did not account for differences in race (which were likely inexistent given the general demographics of the studied population) and other potential group identities of the participants, such as nationality. This potentially represents a limitation to generalizability in some contexts where such group identities are of high importance.
6. CONCLUSIONS
We found a behavioral dimension including in‐group social optimism as well as general optimism‐pessimism measures to be associated with a dimension of within‐network connectivity during a social optimism task. It is noteworthy how similar said patterns are to a prior study focusing on resting‐state connectivity. This similarity indicates that many of the brain and cognitive functions that are present even when people are not focused on anything in particular, are the same processes that support the actual expressions of optimism during social tasks. In other words, these biases appear to be based on inherent properties to the biological system of the human brain, rather than situation specific. The combination of the two studies highlights the importance of networks including the dorsal precuneus and dorsal SMN in terms of extending general personal optimism to biases on in‐groups. We interpret this as lockstep processing in said networks, indicative of a combination of both social and personal optimism. Meanwhile, reduced connectivity in networks involving the IFG, insula and MTG, as well as the connection between midline and salience network structures was associated with reduced social optimism. Based on these observations, we postulate that personal optimism biases tend to extend more towards social optimism biases, if said regions important to cognitive processing are over‐integrated, that is, lack diversity and flexibility between regions that ought to subserve different functions.
FUNDING INFORMATION
The granting body for this work is Swiss National Science Foundation, Grants PP00P1_150492 and PP00P1_183709 awarded to Tatjana Aue, Protocol number: 2015‐10‐000008. Gaelle Eve Doucet is funded by the National Institute of General Medical Sciences Grant P20 GM144641. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
CONFLICT OF INTEREST STATEMENT
The authors confirm that no competing interests exist.
INFORMED CONSENT
All participants gave informed consent prior to participation and data has been anonymized.
Supporting information
Figure S1. Depiction of results for mode 4 of the sCCA. Panel (A): weights greater than 0.2/lower than −0.2 for the optimism variate. (B) Weights greater than 0.2/lower than −0.2 for the network connectivity variate. (C) Scatter plot of the two variates. (D) Visual depiction of the networks and their weights. CEN: central executive network, DMN: default mode network, IFG: inferior frontal gyrus, LOT: life orientation test, MTG: medial temporal gyrus, NW: network, SMN: sensorimotor network.
Figure S2. Depiction of results for mode 5 of the sCCA. Panel (A) Weights greater than 0.2/lower than −0.2 for the optimism variate. (B) Weights greater than 0.2/lower than −0.2 for the network connectivity variate. (C) Scatter plot of the two variates. (D) Visual depiction of the networks and their weights. CEN: central executive network, COS: comparative optimism scale, DMN: default mode network, IFG: inferior frontal gyrus, LOT: life orientation test, MTG: medial temporal gyrus, NW: network.
Figure S3. Depiction of the distribution of Moser's RR score for significant modes.
Table S1A. Behavioral weights of each mode against the within‐network connectivity variate.
Table S1B. Neuroimaging weights of each mode against the optimism variate.
ACKNOWLEDGMENTS
Calculations were performed on UBELIX (http://www.id.unibe.ch/hpc), the HPC cluster at the University of Bern.
Moser, D. A. , Dricu, M. , Kotikalapudi, R. , Doucet, G. E. , & Aue, T. (2023). Within‐network brain connectivity during a social optimism task is related to personal optimism and optimism for in‐group members. Human Brain Mapping, 44(12), 4561–4571. 10.1002/hbm.26400
DATA AVAILABILITY STATEMENT
The underlying data (in preprocessed and anonymized form) will be made available as an excel file with the journal as supplementary material if possible, or if not possible on a public repository. Two‐view sparse canonical correlation analysis (sCCA) for MATLAB, used in accordance with (Ing et al., 2019), is available at github (https://github.com/alexjamesing/mscca-regression-code/blob/master/SCCA_SCCA.m). Permutations for significance testing and reliability analyses were performed with the PALM toolbox (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM) (Winkler et al., 2014). Code for Moser's RR‐score (Moser et al., 2018) is available at: https://github.com/domamo/Matlab-code-and-example-to-calculate-RR-score-as-related-to-Moser-et-al-2018.
REFERENCES
- Aron, A. , Aron, E. N. , & Smollan, D. (1992). Inclusion of other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology, 63(4), 596–612. 10.1037/0022-3514.63.4.596 [DOI] [Google Scholar]
- Aue, T. (2014). I feel good whether my friends win or my foes lose: Brain mechanisms underlying feeling similarity. Neuropsychologia, 60, 159–167. 10.1016/j.neuropsychologia.2014.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aue, T. , Buhrer, S. , Mayer, B. , & Dricu, M. (2021). Empathic responses to social targets: The influence of warmth and competence perceptions, situational valence, and social identification. PLoS One, 16(3), e0248562. 10.1371/journal.pone.0248562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aue, T. , Dricu, M. , Moser, D. A. , Mayer, B. , & Buhrer, S. (2021). Comparing personal and social optimism biases: Magnitude, overlap, modifiability, and links with social identification and expertise. Humanities & Social Sciences Communications, 8(1), 233. 10.1057/s41599-021-00913-8 [DOI] [Google Scholar]
- Aue, T. , Dricu, M. , Singh, L. , Moser, D. A. , & Kotikalapudi, R. (2021). Enhanced sensitivity to optimistic cues is manifested in brain structure: A voxel‐based morphometry study. Social Cognitive and Affective Neuroscience, 16(11), 1170–1181. 10.1093/scan/nsab075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aue, T. , Hoeppli, M. E. , Piguet, C. , Hofstetter, C. , Rieger, S. W. , & Vuilleumier, P. (2015). Brain systems underlying encounter expectancy bias in spider phobia. Cognitive, Affective, & Behavioral Neuroscience, 15(2), 335–348. 10.3758/s13415-015-0339-6 [DOI] [PubMed] [Google Scholar]
- Aue, T. , Nusbaum, H. C. , & Cacioppo, J. T. (2012). Neural correlates of wishful thinking. Social Cognitive and Affective Neuroscience, 7(8), 991–1000. 10.1093/scan/nsr081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi, Y. , Restom, K. , Liau, J. , & Liu, T. T. (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37(1), 90–101. 10.1016/j.neuroimage.2007.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell, A. J. , & Sejnowski, T. J. (1995). An information‐maximization approach to blind separation and blind deconvolution. Neural Computation, 7(6), 1129–1159. 10.1162/neco.1995.7.6.1129 [DOI] [PubMed] [Google Scholar]
- Brooks, J. C. , & Tracey, I. (2007). The insula: A multidimensional integration site for pain. Pain, 128(1–2), 1–2. 10.1016/j.pain.2006.12.025 [DOI] [PubMed] [Google Scholar]
- Calhoun, V. D. , Adali, T. , Pearlson, G. D. , & Pekar, J. J. (2001). A method for making group inferences from functional MRI data using independent component analysis. Human Brain Mapping, 14(3), 140–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun, V. D. , Liu, J. , & Adali, T. (2009). A review of group ICA for fMRI data and ICA for joint inference of imaging, genetic, and ERP data. NeuroImage, 45(1 Suppl), S163–S172. 10.1016/j.neuroimage.2008.10.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna, A. E. , & Trimble, M. R. (2006). The precuneus: A review of its functional anatomy and behavioural correlates. Brain, 129(Pt 3), 564–583. 10.1093/brain/awl004 [DOI] [PubMed] [Google Scholar]
- Conversano, C. , Rotondo, A. , Lensi, E. , Della Vista, O. , Arpone, F. , & Reda, M. A. (2010). Optimism and its impact on mental and physical well‐being. Clinical Practice and Epidemiology in Mental Health, 6, 25–29. 10.2174/1745017901006010025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuddy, A. J. , Glick, P. , & Beninger, A. (2011). The dynamics of warmth and competence judgments, and their outcomes in organizations. Research in Organizational Behavior, 31, 73–98. [Google Scholar]
- Cuddy, A. J. C. , Fiske, S. T. , & Glick, P. (2007). The BIAS map: Behaviors from intergroup affect and stereotypes. Journal of Personality and Social Psychology, 92(4), 631–648. 10.1037/0022-3514.92.4.631 [DOI] [PubMed] [Google Scholar]
- Doucet, G. E. , Lee, W. H. , & Frangou, S. (2019). Evaluation of the spatial variability in the major resting‐state networks across human brain functional atlases. Human Brain Mapping, 40(15), 4577–4587. 10.1002/hbm.24722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dricu, M. , Buhrer, S. , Hesse, F. , Eder, C. , Posada, A. , & Aue, T. (2018). Warmth and competence predict overoptimistic beliefs for out‐group but not in‐group members. PLoS One, 13(11), e0207670. 10.1371/journal.pone.0207670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dricu, M. , Kress, L. , & Aue, T. (2020). The neurophysiological basis of optimism bias. In Aue T. & Okon‐Singer H. (Eds.), Cognitive biases in health and psychiatric disorders: Neurophysiological foundations. Elsevier. [Google Scholar]
- Dricu, M. , Schupbach, L. , Bristle, M. , Wiest, R. , Moser, D. A. , & Aue, T. (2020). Group membership dictates the neural correlates of social optimism biases. Scientific Reports, 10(1), 1139. 10.1038/s41598-020-58121-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske, S. T. (2015). Intergroup biases: A focus on stereotype content. Current Opinion in Behavioral Sciences, 3, 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiske, S. T. (2017). Prejudices in cultural contexts: Shared stereotypes (gender, age) versus variable stereotypes (race, ethnicity, religion). Perspectives on Psychological Science, 12(5), 791–799. 10.1177/1745691617708204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston, K. J. , Williams, S. , Howard, R. , Frackowiak, R. S. , & Turner, R. (1996). Movement‐related effects in fMRI time‐series. Magnetic Resonance in Medicine, 35(3), 346–355. 10.1002/mrm.1910350312 [DOI] [PubMed] [Google Scholar]
- Glaesmer, H. , Hoyer, J. , Klotsche, J. , & Herzberg, P. Y. (2008). Die deutsche Version des Life‐Orientation‐Test (LOT‐R) zum dispositionellen Optimismus und Pessimismus. Zeitschrift für Gesundheitspsychologie, 16, 26–31. [Google Scholar]
- Hahn, B. , Ross, T. J. , & Stein, E. A. (2006). Neuroanatomical dissociation between bottom‐up and top‐down processes of visuospatial selective attention. NeuroImage, 32(2), 842–853. 10.1016/j.neuroimage.2006.04.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlin, B. , Navarro, V. , & Dupont, S. (2021). The temporal pole: From anatomy to function—A literature appraisal. Journal of Chemical Neuroanatomy, 113, 101925. 10.1016/j.jchemneu.2021.101925 [DOI] [PubMed] [Google Scholar]
- Himberg, J. , Hyvarinen, A. , & Esposito, F. (2004). Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage, 22(3), 1214–1222. 10.1016/j.neuroimage.2004.03.027 [DOI] [PubMed] [Google Scholar]
- Ing, A. , Samann, P. G. , Chu, C. , Tay, N. , Biondo, F. , Robert, G. , Jia, T. , Wolfers, T. , Desrivières, S. , Banaschewski, T. , Bokde, A. L. W. , Bromberg, U. , Büchel, C. , Conrod, P. , Fadai, T. , Flor, H. , Frouin, V. , Garavan, H. , Spechler, P. A. , … IMAGEN Consortium . (2019). Identification of neurobehavioural symptom groups based on shared brain mechanisms. Nature Human Behaviour, 3(12), 1306–1318. 10.1038/s41562-019-0738-8 [DOI] [PubMed] [Google Scholar]
- Knyazev, G. G. , Savostyanov, A. N. , Bocharov, A. V. , & Rudych, P. D. (2021). How self‐appraisal is mediated by the brain. Frontiers in Human Neuroscience, 15, 700046. 10.3389/fnhum.2021.700046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress, L. , & Aue, T. (2017). The link between optimism bias and attention bias: A neurocognitive perspective. Neuroscience and Biobehavioral Reviews, 80, 688–702. 10.1016/j.neubiorev.2017.07.016 [DOI] [PubMed] [Google Scholar]
- Kress, L. , & Aue, T. (2019). Learning to look at the bright side of life: Attention bias modification training enhances optimism bias. Frontiers in Human Neuroscience, 13, 222. 10.3389/fnhum.2019.00222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress, L. , Bristle, M. , & Aue, T. (2018). Seeing through rose‐colored glasses: How optimistic expectancies guide visual attention. PLoS One, 13(2), e0193311. 10.1371/journal.pone.0193311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzmanovic, B. , Jefferson, A. , & Vogeley, K. (2016). The role of the neural reward circuitry in self‐referential optimistic belief updates. NeuroImage, 133, 151–162. 10.1016/j.neuroimage.2016.02.014 [DOI] [PubMed] [Google Scholar]
- Ligeza, T. S. , Wyczesany, M. , Tymorek, A. D. , & Kaminski, M. (2016). Interactions between the prefrontal cortex and attentional systems during volitional affective regulation: An effective connectivity reappraisal study. Brain Topography, 29(2), 253–261. 10.1007/s10548-015-0454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monosov, I. E. (2017). Anterior cingulate is a source of valence‐specific information about value and uncertainty. Nature Communications, 8(1), 134. 10.1038/s41467-017-00072-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D. A. (2018). Matlab code and example to calculate RR‐score as related to Moser et al 2018 . 10.13140/RG.2.2.33544.26883 [DOI]
- Moser, D. A. , Doucet, G. E. , Lee, W. H. , Rasgon, A. , Krinsky, H. , Leibu, E. , Ing, A. , Schumann, G. , Rasgon, N. , & Frangou, S. (2018). Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry, 75(4), 386–395. 10.1001/jamapsychiatry.2017.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D. A. , Dricu, M. , Kotikalapudi, R. , Doucet, G. E. , & Aue, T. (2021). Reduced network integration in default mode and executive networks is associated with social and personal optimism biases. Human Brain Mapping, 42(9), 2893–2906. 10.1002/hbm.25411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, D. A. , Dricu, M. , Wiest, R. , Schupbach, L. , & Aue, T. (2020). Social optimism biases are associated with cortical thickness. Social Cognitive and Affective Neuroscience, 15(7), 745–754. 10.1093/scan/nsaa095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moutsiana, C. , Charpentier, C. J. , Garrett, N. , Cohen, M. X. , & Sharot, T. (2015). Human frontal‐subcortical circuit and asymmetric belief updating. The Journal of Neuroscience, 35(42), 14077–14085. 10.1523/JNEUROSCI.1120-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner, K. N. , Knierim, K. , Ludlow, D. H. , Hanelin, J. , Ramachandran, T. , Glover, G. , & Mackey, S. C. (2004). Reflecting upon feelings: An fMRI study of neural systems supporting the attribution of emotion to self and other. Journal of Cognitive Neuroscience, 16(10), 1746–1772. 10.1162/0898929042947829 [DOI] [PubMed] [Google Scholar]
- Owen, A. M. , McMillan, K. M. , Laird, A. R. , & Bullmore, E. (2005). N‐back working memory paradigm: A meta‐analysis of normative functional neuroimaging studies. Human Brain Mapping, 25(1), 46–59. 10.1002/hbm.20131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyediran, O. A. , Rivas, M. F. , Coulson, M. , & Kernohan, D. (2018). Cooperation and optimism in a social dilemma. Bulletin of Economic Research, 70(4), 335–340. 10.1111/boer.12161 [DOI] [Google Scholar]
- Pineda, J. A. (2008). Sensorimotor cortex as a critical component of an ‘extended’ mirror neuron system: Does it solve the development, correspondence, and control problems in mirroring? Behavioral and Brain Functions, 4, 47. 10.1186/1744-9081-4-47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesque, F. , & Brass, M. (2019). The role of the temporoparietal junction in self‐other distinction. Brain Topography, 32(6), 943–955. 10.1007/s10548-019-00737-5 [DOI] [PubMed] [Google Scholar]
- Rasmussen, H. N. , Scheier, M. F. , & Greenhouse, J. B. (2009). Optimism and physical health: A meta‐analytic review. Annals of Behavioral Medicine, 37(3), 239–256. 10.1007/s12160-009-9111-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg, M. (1965). Society and the adolescent selfimage. Princeton University Press. [Google Scholar]
- Scheier, M. F. , Carver, C. S. , & Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self‐mastery, and self‐esteem) – A reevaluation of the life orientation test. Journal of Personality and Social Psychology, 67(6), 1063–1078. 10.1037/0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Segerdahl, A. R. , Mezue, M. , Okell, T. W. , Farrar, J. T. , & Tracey, I. (2015). The dorsal posterior insula subserves a fundamental role in human pain. Nature Neuroscience, 18(4), 499–500. 10.1038/nn.3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkurko, A. V. (2013). Is social categorization based on relational ingroup/outgroup opposition? A meta‐analysis. Social Cognitive and Affective Neuroscience, 8(8), 870–877. 10.1093/scan/nss085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, L. , Schupbach, L. , Moser, D. A. , Wiest, R. , Hermans, E. J. , & Aue, T. (2020). The effect of optimistic expectancies on attention bias: Neural and behavioral correlates. Scientific Reports, 10(1), 6495. 10.1038/s41598-020-61440-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S. M. , Fox, P. T. , Miller, K. L. , Glahn, D. C. , Fox, P. M. , Mackay, C. E. , Filippini, N. , Watkins, K. E. , Toro, R. , Laird, A. R. , & Beckmann, C. F. (2009). Correspondence of the brain's functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America, 106(31), 13040–13045. 10.1073/pnas.0905267106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speitel, C. , Traut‐Mattausch, E. , & Jonas, E. (2019). Functions of the right DLPFC and right TPJ in proposers and responders in the ultimatum game. Social Cognitive and Affective Neuroscience, 14(3), 263–270. 10.1093/scan/nsz005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbeis, N. (2016). The role of self‐other distinction in understanding others' mental and emotional states: Neurocognitive mechanisms in children and adults. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 371(1686), 20150074. 10.1098/rstb.2015.0074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viinikainen, M. , Jaaskelainen, I. P. , Alexandrov, Y. , Balk, M. H. , Autti, T. , & Sams, M. (2010). Nonlinear relationship between emotional valence and brain activity: Evidence of separate negative and positive valence dimensions. Human Brain Mapping, 31(7), 1030–1040. 10.1002/hbm.20915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeley, K. , May, M. , Ritzl, A. , Falkai, P. , Zilles, K. , & Fink, G. R. (2004). Neural correlates of first‐person perspective as one constituent of human self‐consciousness. Journal of Cognitive Neuroscience, 16(5), 817–827. 10.1162/089892904970799 [DOI] [PubMed] [Google Scholar]
- Von Collani, G. , & Herzberg, Y. (2003). Eine revidierte Fassung der deutschsprachigen Skala zum Selbstwertgefühl von Rosenberg. Zeitschrift für Differentielle Und Diagnostische Psychologie, 24(1), 3–7. [Google Scholar]
- Weinstein, N. D. (1980). Unrealistic optimism about future life events. Journal of Personality and Social Psychology, 39, 806–820. 10.1037/0022-3514.39.5.806 [DOI] [Google Scholar]
- Williams, J. H. G. , Huggins, C. F. , Zupan, B. , Willis, M. , Van Rheenen, T. E. , Sato, W. , Palermo, R. , Ortner, C. , Krippl, M. , Kret, M. , Dickson, J. M. , Li, C.‐s. R. , & Lowe, L. (2020). A sensorimotor control framework for understanding emotional communication and regulation. Neuroscience and Biobehavioral Reviews, 112, 503–518. 10.1016/j.neubiorev.2020.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windschitl, P. D. , & Stuart, J. O. R. (2015). Optimism biases. In The Wiley Blackwell handbook of judgment and decision making (pp. 431–455). West Sussex, UK. [Google Scholar]
- Winkler, A. M. , Ridgway, G. R. , Webster, M. A. , Smith, S. M. , & Nichols, T. E. (2014). Permutation inference for the general linear model. NeuroImage, 92, 381–397. 10.1016/j.neuroimage.2014.01.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten, D. M. , Tibshirani, R. , & Hastie, T. (2009). A penalized matrix decomposition, with applications to sparse principal components and canonical correlation analysis. Biostatistics, 10(3), 515–534. 10.1093/biostatistics/kxp008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan, C. G. , Wang, X. D. , Zuo, X. N. , & Zang, Y. F. (2016). DPABI: Data Processing & Analysis for (resting‐state) brain imaging. Neuroinformatics, 14(3), 339–351. 10.1007/s12021-016-9299-4 [DOI] [PubMed] [Google Scholar]
- Zhuang, X. , Yang, Z. , & Cordes, D. (2020). A technical review of canonical correlation analysis for neuroscience applications. Human Brain Mapping, 41(13), 3807–3833. 10.1002/hbm.25090 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Depiction of results for mode 4 of the sCCA. Panel (A): weights greater than 0.2/lower than −0.2 for the optimism variate. (B) Weights greater than 0.2/lower than −0.2 for the network connectivity variate. (C) Scatter plot of the two variates. (D) Visual depiction of the networks and their weights. CEN: central executive network, DMN: default mode network, IFG: inferior frontal gyrus, LOT: life orientation test, MTG: medial temporal gyrus, NW: network, SMN: sensorimotor network.
Figure S2. Depiction of results for mode 5 of the sCCA. Panel (A) Weights greater than 0.2/lower than −0.2 for the optimism variate. (B) Weights greater than 0.2/lower than −0.2 for the network connectivity variate. (C) Scatter plot of the two variates. (D) Visual depiction of the networks and their weights. CEN: central executive network, COS: comparative optimism scale, DMN: default mode network, IFG: inferior frontal gyrus, LOT: life orientation test, MTG: medial temporal gyrus, NW: network.
Figure S3. Depiction of the distribution of Moser's RR score for significant modes.
Table S1A. Behavioral weights of each mode against the within‐network connectivity variate.
Table S1B. Neuroimaging weights of each mode against the optimism variate.
Data Availability Statement
The underlying data (in preprocessed and anonymized form) will be made available as an excel file with the journal as supplementary material if possible, or if not possible on a public repository. Two‐view sparse canonical correlation analysis (sCCA) for MATLAB, used in accordance with (Ing et al., 2019), is available at github (https://github.com/alexjamesing/mscca-regression-code/blob/master/SCCA_SCCA.m). Permutations for significance testing and reliability analyses were performed with the PALM toolbox (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/PALM) (Winkler et al., 2014). Code for Moser's RR‐score (Moser et al., 2018) is available at: https://github.com/domamo/Matlab-code-and-example-to-calculate-RR-score-as-related-to-Moser-et-al-2018.


