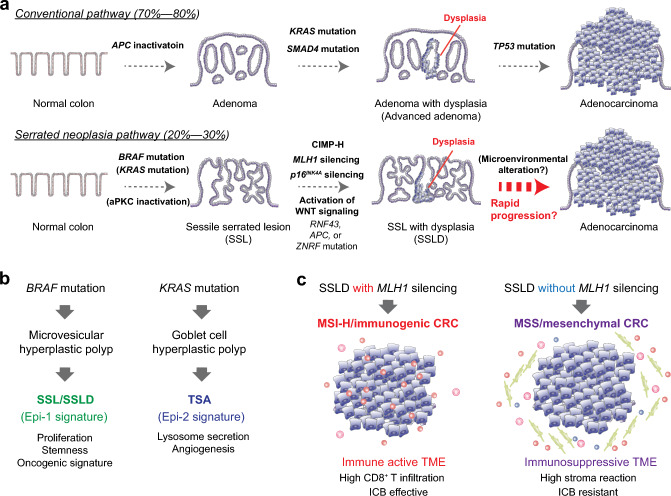
Fig. 3.
Molecular evolution of sessile serrated lesion with dysplasia (SSLD) and SSLD-derived cancer. a Colorectal cancer (CRC) develops through two distinctive pathways. Conventional pathway (top), accounting for 70%–80% of sporadic CRCs, is initiated by inactivation of APC in normal cells which results in the formation of conventional-type adenoma. Conventional adenoma acquires the additional mutations of KRAS, SMAD4 and TP53 which results in the progression to adenoma with dysplasia (advanced adenoma) and finally to adenocarcinoma. Serrated neoplasia pathway (bottom), accounting for 20%–30% of CRCs, is initiated mostly by BRAF mutation, which results in the formation of sessile serrated lesion (SSL). Hypermethylation in the CpG island promoter regions (CpG island methylation phenotype; CIMP) of tumor suppressors such as MLH1 and p16INK4a results in the silencing of these genes and allow a progression of SSL to SSLD. This process is also associated with the activation of WNT signaling pathway, led by mutation of RNF43, APC or ZNRF. Epigenetic silencing of tumor suppressors and activation of WNT signaling, together with microenvironmental alteration, induces rapid progression of SSLD to adenocarcinoma. b BRAF mutated lesions develop into SSL/SSLD via microvesicular hyperplastic polyp, while KRAS mutated lesions are more likely to develop into traditional serrated adenoma (TSA) via goblet cell hyperplastic polyp. Epi-1 signature is mainly observed in SSL and SSLD, while Epi-2 is exclusively in TSA. Compared to Epi-2, that is characterized by upregulated pathways of lysosome secretion and angiogenesis, Epi-1 demonstrates higher expression of genes related to proliferation, stemness, and oncogenic signatures. c Presumable progression of SSLD to CRC, considering their microsatellite status and tumor microenvironment (TME). BRAF-mutant SSLD with epigenetic silencing of MLH1 progress to immunogenic microsatellite instability high (MSI-H) CRC, whereas SSLD without loss of MLH1 progress to microsatellite stable (MSS) CRC. MSI-H CRC is associated with an immune-active TME and responds better to immune checkpoint blockage (ICB) therapy. MSS CRC of serrated origin is mesenchymal and exhibits high stroma reaction, ICB-resistance, and metastatic behavior with poor prognosis