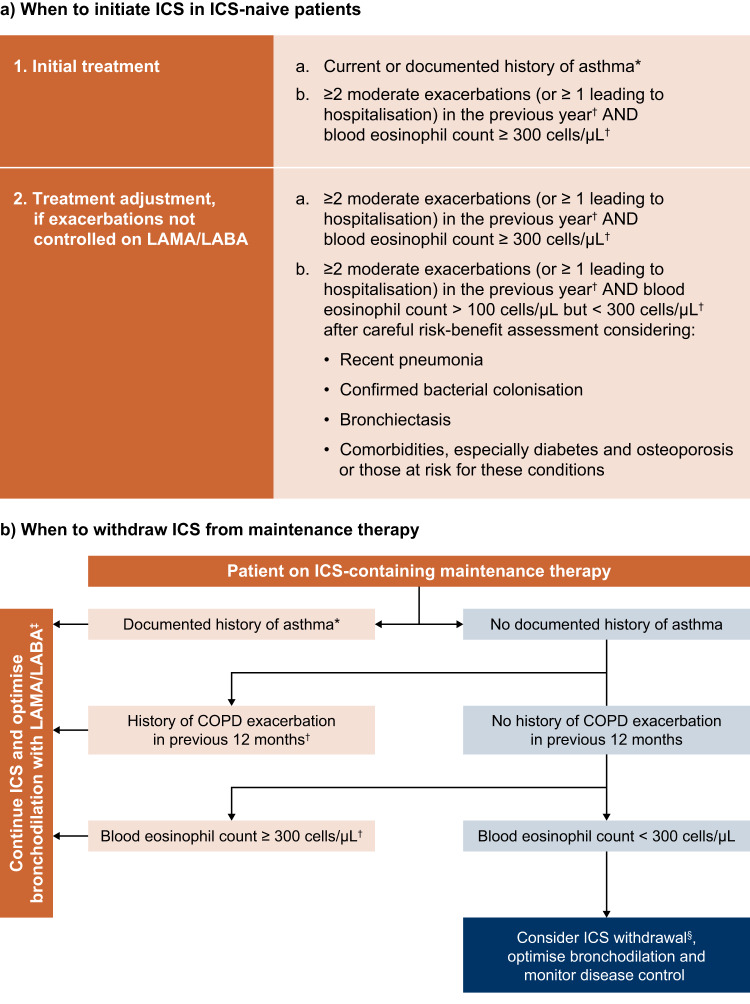
Fig. 3. Practical guide to prescribing ICS for the treatment of COPD.
Adapted from the International Primary Care Respiratory Group (IPCRG) desktop helper for the appropriate use and withdrawal of ICS, 2020. Available at link. *This may include asthmatic features/features suggesting steroid responsiveness, including any previous, secure diagnosis of asthma or atopy, a higher blood eosinophil count, substantial variation in FEV1 over time (at least 400 ml) or substantial diurnal variation in peak expiratory flow (at least 20%). †Or since previous assessment if less than 12 months. ‡For patients with exacerbations despite triple therapy (LAMA/LABA/ICS), consider add-on therapy with roflumilast or macrolides. §If blood eosinophil count is 150–300 cells/μl, reduce ICS dose/switch to an ICS with a better safety profile. If blood eosinophil count is <150 cells/µl, and there is no/questionable asthma history or exacerbation in the previous 12 months, consider withdrawal as risks of ICS are likely to outweigh any benefit. COPD chronic obstructive pulmonary disease, FEV1 forced expiratory volume in 1 s, ICS inhaled corticosteroids, LABA long-acting β2-agonist, LAMA long-acting muscarinic antagonist.