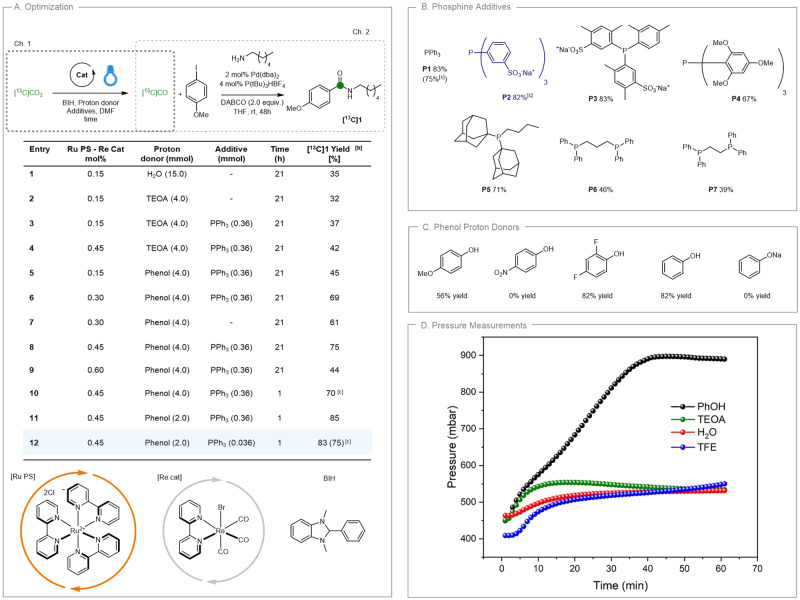
Fig. 2. Optimisation of the CO2-to-CO reduction coupled with aminocarbonylation.
A Optimisation of the transformation. See Supplementary Tables 1 and 2, for full experimental details. [a] Ch.1: [13C]CO2 (0.365 mmol), BIH (0.78 mmol); DMF, room temperature; Ch.2: 4‐iodoanisole (0.724 mmol), n‐hexylamine (1.45 mmol), DABCO (1.45 mmol), Pd(dba)2 (2 mol%), P(tBu)3HBF4 (4 mol%), THF (0.24 M), 25 °C, 48 h. [b] 1H-NMR yields calculated using 1,3,5-trimethoxybenzene as internal standard. [c] Yield of isolated product. DMF dimethylformamide, THF tetrahydrofuran, BIH 1,3-dimethyl-2-phenylbenzimidazoline, TEOA triethanolamine, PPh3 triphenylphosphine, DABCO 1,4-diazabicyclo[2.2.2]octane, Pd(dba)2 Palladium(0) bis(dibenzylideneacetone), P(tBu)3HBF4 Tri-tert-butylphosphine tetrafluoroborate. B Screening of phosphine additives: conditions reported in Entry 12 (A) were used (0.036 mmol). C Screening of phenol proton donors: phosphine P2 was used (0.036 mmol). D Pressure measurements relating to the rate of CO formation. Conditions: [13C]CO2 (0.40 mmol), BIH (0.87 mmol); proton source (2.22 mmol); Ru PS (0.45 mol%); Re cat (0.45 mol%) in DMF. The reaction mixture was stirred at room temperature under blue light irradiation (low intensity) for 1 h. TEOA triethanolamine, TFE trifluoroethanol.