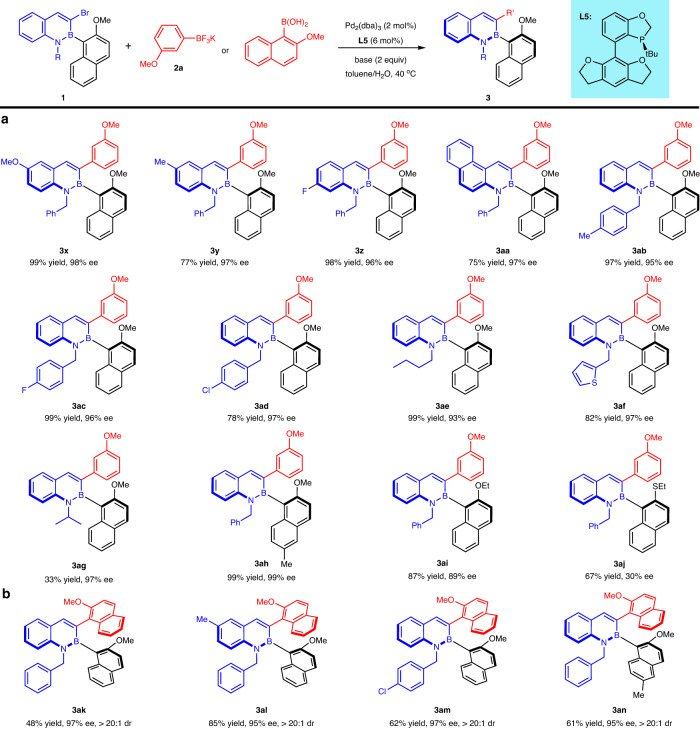
Fig. 5. Substrate scope for racemic 3-bromo-2,1-borazaronaphthalenes and diaxially chiral compounds.
a Scope for racemic 3-bromo-2,1-borazaronaphthalenes. Reaction conditions: 1 (0.1 mmol), 2a (0.13 mmol), Pd2(dba)3 (2 mol%), L5 (6 mol%), NaHCO3 (2 equiv) in toluene (1.5 mL)/H2O (0.3 mL) at 40 °C; isolated yields are provided. b Scope of adjacent C-B and C-C diaxially chiral compounds. Reaction conditions: 1 (0.1 mmol), 2-methoxy-1-naphthyl)boronic acid (1.3–4.0 equiv), Pd2(dba)3 (2 mol%), L5 (6 mol%), Li2CO3 (2.0–4.0 equiv) in toluene (1.5 mL)/H2O (0.15 mL) at 40 °C; isolated yields are provided.