Figure 2. Desynchronization of evoked neurotransmitter release.
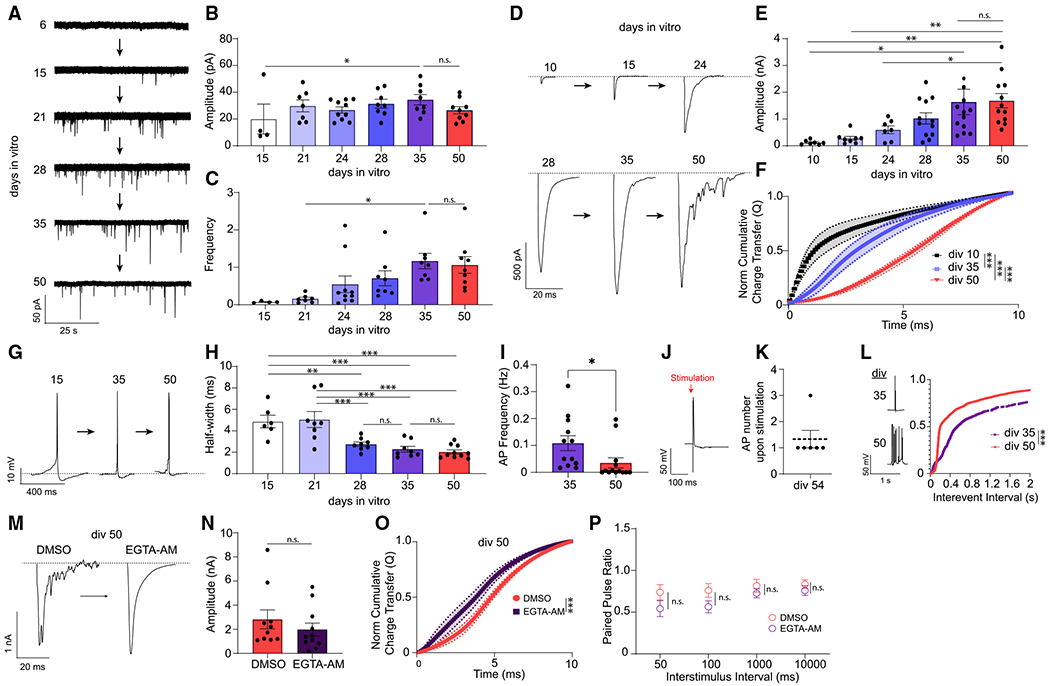
(A) mEPSCs representative traces from human iN cells from days in vitro 6 (div6) to div50.
(B) mEPSC amplitudes shows a significant difference between div15 and div35 but do not change from div21 to div50; dots show individual cells; bars show mean ± SEM; div15, n = 4; div21, n = 7; div24, n = 10; div28, n = 8; div35, n = 8; div50, n = 9; N = 2; p = 0.0423 div15 versus div35; p > 0.99, div35 versus div50; one-way ANOVA-Fisher’s LSD.
(C) mEPSC frequency increases from div21 to div35 and does not change from div35 to div50; dots show individual cells; bars show mean ± SEM; div15, n = 4; div21, n = 7; div24, n = 10; div28, n = 8; div35, n = 8; div50, n = 9;N = 2; p = 0.044 div21 versus div35; p = 0.819, div35 versus div50; one-way ANOVA-Fisher’s LSD.
(D) eEPSC representative traces from div10 to div50.
(E) eEPSC amplitudes increase from div10 to div35 but do not change from div35 to div50; dots show individual cells; bars show mean ± SEM; div10, n = 7; div15, n = 8; div24, n = 7; div28, n = 12; div35, n = 12; div50, n = 12; N = 2; p = 0.0219, div10 versus div35; p = 0.0011,div10 versus div50; p = 0.0021,div15 versus div50; p = 0.0207, div24 versus div50; p = 0.2203, div35 versus div50; one-way ANOVA-Fisher’s LSD.
(F) The NCCT(Q) of eEPSCs at div10, div28, and div50 demonstrating the progressive desynchronization of evoked neurotransmitter release upon in vitro aging; bars show mean ± SEM; div10, n = 6; div28, n = 8; div50, n = 10; N = 2; p < 0.0001; Kolmogorov-Smirnov test; p < 0.001; simple linear regression.
(G) AP representative traces at div10, div35, and div50.
(H) AP half-width decreases as iN cells mature from div15 to div35 but does not change from div35 to div50; dots show individual cells; bars show mean ± SEM; div15, n = 6; div21, n = 8; div28, n = 8; div35, n = 8; div50, n = 10; N = 2; p = 0.003, div15 versus div28; p < 0.001, div15 versus div35; p < 0.001, div15 versus div50; p < 0.001, div21 versus div28; p < 0.001, div21 versus div35; p < 0.001, div21 versus div50; p = 0.446, div28 versus div35; p = 0.661, div35 versus div50; one-way ANOVA-Fisher’s LSD.
(I) Spontaneous AP frequency decreases significantly from div35 to div50 in human iN cells; dots show individual cells; bars show mean ± SEM; div35, n = 12; div50, n = 13; N = 2; p = 0.037; unpaired t test.
(J and K) At div54, upon stimulation of human iN cells we observed single APs 1,000 ms following each stimulation; dots show individual cells; bars show mean ± SEM; n = 6; N = 1.
(L) The interevent intervals (ms) cumulative histogram of APs at div35 and div50 iN cells are different revealing a burst-like firing pattern at div50; bars show mean ± SEM; div35, n = 9; div50, n = 8; N = 2; p < 0.001; simple linear regression; p < 0.0001; Kolmogorov-Smirnov test.
(M) Representative traces of eEPSCs following acute (15 min) DMSO or EGTA-AM treatment at div50.
(N and P) EGTA-AM treatment does not significantly change eEPSC amplitude or PPRs; dots show individual cells; bars show mean ± SEM; eEPSC amplitude, DMSO, n = 10; EGTA-AM, n = 11; N = 2; p = 0.378; unpaired t test; PPR, 50 ms, DMSO, n = 8; EGTA-AM, n = 8; N = 2; p = 0.161; unpaired t test; 100 ms, DMSO, n = 8, EGTA-AM, n = 8; N = 2; p = 0.09; unpaired t test; 1,000 ms, DMSO, n = 9, EGTA-AM, n = 9;N = 2; p = 0.34; unpaired t test; 10,000 ms, DMSO, n = 9, EGTA-AM, n = 10; N = 2; p = 0.35; unpaired t test.
(O) NCCT of eEPSCs following acute (15 min) DMSO or EGTA-AM treatment at div50 reveal that EGTA-AM treatment makes eEPSCs significantly more synchronous; bars show mean ± SEM; DMSO, n = 10; EGTA-AM, n = 11; N = 2; p < 0.001; simple linear regression; p < 0.0001; Kolmogorov-Smirnov test. Significance levels are as follows: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001; ns, non-significance. See also Figure S2 and Table S1.
