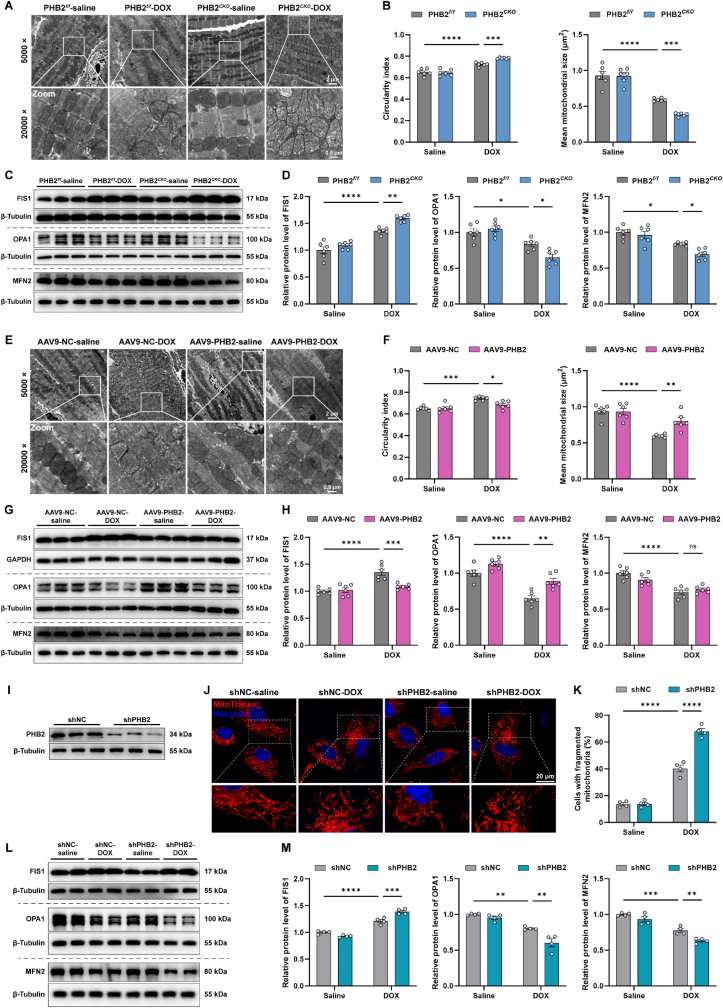
Fig. 3.
PHB2 deficiency aggravated DOX-induced mitochondrial fragmentation in mouse hearts.
(A and B) Representative images of mitochondrial ultrastructure using TEM in myocardial sections from PHB2flox/flox and PHB2CKO mice, and quantification of circularity index and mitochondrial size based on TEM images (n = 6 per group). (C and D) Representative immunoblotting images and quantification of FIS1, OPA1, and MFN2 in heart lysates from PHB2flox/flox and PHB2CKO mice (β-Tubulin used as an internal control, n = 6 per group). (E and F) Representative images of mitochondrial ultrastructure using TEM in myocardial sections from AAV9-NC and AAV9-PHB2 mice, and quantification of circularity index and mitochondrial size based on TEM images (n = 6 per group). (G and H) Representative immunoblotting images and quantification of FIS1, OPA1, and MFN2 in heart lysates from AAV9-NC and AAV9-PHB2 mice (GAPDH and β-Tubulin used as internal controls, n = 6 per group). (I) Representative immunoblotting images of PHB2 knockdown using adenoviruses carrying shRNA in primary cardiomyocytes (β-Tubulin used as an internal control). (J and K) Representative images of MitoTracker staining in neonatal rat cardiomyocytes (NRCMs) with and without DOX challenge (0.1 μM) for 24 h after adenovirus transfection, and quantification of the percentage of cells with fragmented mitochondria (those with >50% fragmented mitochondria, n = 4 independent experiments per group and approximately 20 cells for each experiment). (L and M) Representative immunoblotting images and quantification of FIS1, OPA1, and MFN2 in primary cardiomyocytes with and without DOX challenge for 24 h after adenovirus transfection (β-Tubulin used as an internal control, n = 4 independent experiments per group). Mean ± SEM. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, ns, no significance. For statistical analysis, two-way ANOVA with Tukey's test for multiple comparisons was used.